The role of bud bank in glyphosate tolerance of two herbaceous species
DOI:
https://doi.org/10.31055/1851.2372.v54.n4.24301Palavras-chave:
Commelina erecta, Eustachys retusa, regeneration, weeds, herbicideResumo
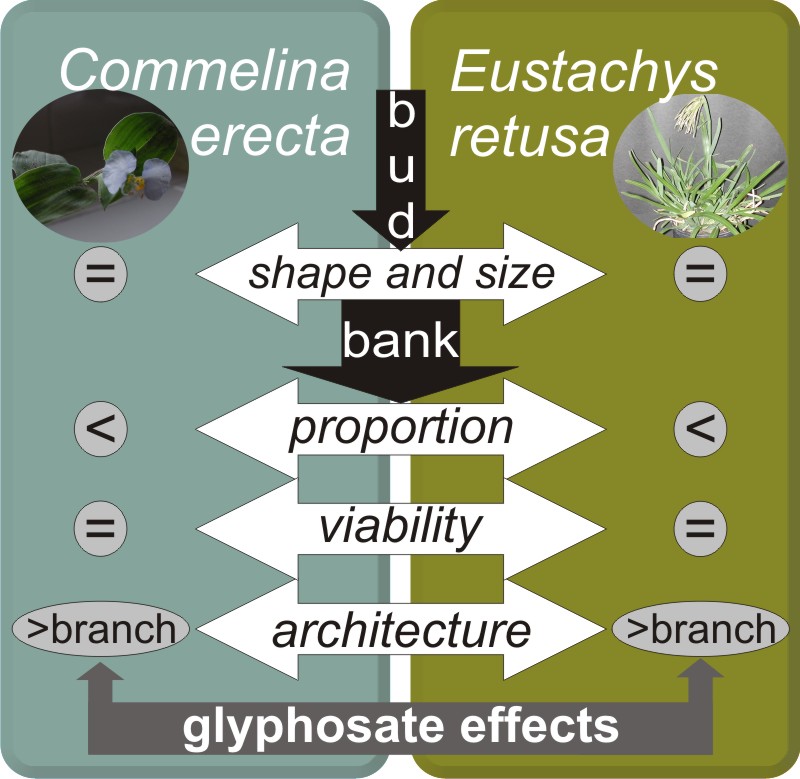
Background and aims: Commelina erecta and Eutsachys retusa are two perennial weeds, which show high resprouting after glyphosate application. This behavior represents a serious problem for weed management. The purpose of this study was to characterize the bud bank of both species and to assess their response to glyphosate application.
M&M: We analyzed 120 reproductive shoots of C. erecta, and 80 reproductive shoots of E. retusa, at 30- and 60-days post- herbicide application. The doses applied to C. erecta were 0 (control), 1.200 and 2.400 g a.i. ha -1 , and to E. retusa were 0 (control), 480 and 1200 g a.i. ha -1 .
Results: We found that both species presented active buds in approximately 50% of their nodes, even after herbicide application. Bud bank dynamics changed in both weeds after herbicide application, and therefore their growth pattern. The activation of originally inhibited buds allowed weeds to regrow and survive after glyphosate application altering their architecture.
Conclusions: The bud bank plays an important role in glyphosate tolerance in both weeds. The resprouting capacity in both species was similar for any dose of glyphosate applied. Therefore, an alternative control strategy based on the increase of the dose of herbicide would not be a successful alternative for the management of these weeds. The interruption of the storage of reserves in the rhizome system and the reduction of the number of buds would be key to effective long-term management of these and other perennial weeds in no tillage cropping system.
Referências
BOND, W. J. & J. J. MIDGLEY. 2001. Ecology of sprouting in woody plants: the persistence niche. Trends Ecol. Evol. 16: 45-51. https://doi.org/10.1016/S0169-5347(00)02033-4
BONSER, S. P. & L. W. AARSSEN. 2009. Interpreting reproductive allometry: Individual strategies of allocation explain size-dependent reproduction in plant populations. Perspect. Pl. Ecol. Evol. Syst. 1: 31-40. https://doi.org/10.1016/j.ppees.2008.10.003
BOSTRÖM, U., L. ANDERSSON, J. FORKMAN, I. HAKMAN, J. LIEW & E. MAGNUSKI. 2013. Seasonal variation in sprouting capacity from intact rhizome systems of three perennial weeds. Weed Res. 53: 387–398. https://doi.org/10.1111/wre.12035
BURKART, A. 1969. Flora Ilustrada de Entre Ríos (Argentina). T. VI, Parte II. Colección Científica del INTA, Buenos Aires.
BUSSO, C. A., R. J. MUELLER & J. H. RICHARDS. 1989. Effects of drought and defoliation on bud viability in two caespitose grasses. Ann. Bot. 63: 477-485. https://doi.org/10.1093/oxfordjournals.aob.a087768
BUSSO, C. A., J. H. RICHARDS & N. J. CHATTERTON. 1990. Nonstructural carbohydrates and spring regrowth of two cool-season grasses: Interaction of drought and clipping. J. Range. Managem. 43: 336-343. https://doi.org/10.2307/3898928
BUSSO, C. A., C. GITTINS, G. F. BECKER & L. GHERMANDI. 2011. Tiller hierarchy and defoliation frequency determine bud viability in the grass Poa ligularis. Ecol. Res. 26: 985-997. https://doi.org/10.1007/s11284-011-0857-9
CASAFE (Cámara de Sanidad Agropecuaria y Fertilizantes- República Argentina). 2017. Guía de productos fitosanitarios para la República Argentina. 18th ed. Buenos Aires, Argentina
DELLAFERRERA, I. M., N. J. GUARISE & A. AMSLER. 2007. Relevamiento de malezas en cultivos de soja en sistema de siembra directa con glifosato del departamento San Justo (Provincia de Santa Fe). Revista FAVE –Sección Agrarias 5/6: 15-25. https://doi.org/10.14409/fa.v5i1/2.1318
DELLAFERRERA, I. M., E. S. PANIGO, F. GONZALEZ-TORRALBA, R. A. DE PRADO, P. J. CHRISTOFFOLETI & M. G. PERRETA. 2015. Características estructurales y fisiológicas de Petunia axillaris relacionadas con su baja sensibilidad a glifosato. Revista Pl. Danin 33: 451-462. https://doi.org/10.1590/S0100-83582015000300008
DEMETRIO, G. R., F. F. COELHO & M. E. BARBOSA. 2014. Body size and clonality consequences for sexual reproduction in a perennial herb of Brazilian rupestrian grasslands. Brazil. J. Biol 74: 744-749. https://doi.org/10.1590/bjb.2014.0070
DENG, Z., X. CHEN, Y. XIE, X. LI, Y. PAN & F. LI. 2013. Effects of size and vertical distribution of buds on sprouting and plant growth of the clonal emergent macrophyte Miscanthus sacchariflorus (Poaceae). Aquatic Bot. 104: 121-126. https://doi.org/10.1016/j.aquabot.2012.08.004
FOURCAUD, T., X. ZHANG, A. STOKES, H. LAMBERS & C. KÖRNER. 2008. Plant Growth Modelling and Applications: The Increasing Importance of Plant Architecture in Growth Models. Ann. Bot.101: 1053-1063. https://doi.org/10.1093/aob/mcn050
HARPER, J. L. 1977. Population biology of plants. 1st ed. Academic Press, Lodon.
HENDRICKSON, J. R. & D. D. BRISKE. 1997. Axillary bud banks of two semiarid perennial grasses: occurrence, longevity, and contribution to population persistence. Oecologia 110: 584-591. https://doi.org/10.1007/s004420050199
HOTHORN, T., F. BRETZ & P. WESTFALL. 2008. Simultaneous inference in general parametric models. Biometr. J. 50: 346-363. https://doi.org/10.1002/bimj.200810425
KLIMEŠOVÁ, J. & L. KLIMEŠ. 2003. Resprouting of herbs in disturbed habitats: is it adequately described by Bellingham–Sparrow’s model? OIKOS 103: 225-229. https://doi.org/10.1034/j.1600-0706.2003.12725.x
KLIMEŠOVÁ, J. & J. MARTÍNKOVÁ. 2004. Intermediate growth forms as a model for the study of plant clonality functioning: an example with root sprouters. Evol Ecol 18: 669-681. https://doi.org/10.1007/s10682-004-5149-1
KLIMEŠOVÁ, J. & L. KLIMEŠ. 2007. Bud banks and their role in vegetative regeneration– A literature review and proposal for simple classification and assessment. Perspect. Pl. Ecol. Evol. Syst 8: 115-129. https://doi.org/10.1016/j.ppees.2006.10.002
KLIMEŠOVÁ, J., V. LATZEL, F. DE BELLO & J. M. VAN GROENENDAEL. 2008. Plant functional traits in studies of vegetation changes in response to grazing and mowing: towards a use of more specific traits. Preslia 80: 245-253.
MALPASSI, R. 2005. Efectos de la aplicación de quizalofop sobre la arquitectura y anatomía foliar de Eleusine indica. Agriscientia 22: 55-62. http://dx.doi.org/10.31047/1668.298x.v22.n2
MOREIRA, B., J. TORMO & J. G. PAUSAS. 2012. To resprout or not to resprout: factors driving intraspecific variability in resprouting. Oikos 121: 1577-1584. https://doi.org/10.1111/j.1600-0706.2011.20258.x
NISENSOHN, L., D. TUESCA, D. FACCINI, E. PURICELLI & J. VITTA. 2011. Factores biológicos que determinan la competencia de Commelina erecta con otras malezas en sistemas de cultivo. Revista Pl. Danin 29: 97-106. https://doi.org/10.1590/S0100-83582011000100012
OTT, J. P. & D. C. HARTNETT. 2015. Bud-bank and tiller dynamics of co-occurring C3 caespitose grasses in mixed-grass prairie. Amer. J. Bot. 102: 1462-1471. https://doi.org/10.3732/ajb.1500039
PANIGO, E. S., I. M. DELLAFERRERA, J. M. ACOSTA, A. G. BENDER, J. I. GARETTO & M. G. PERRETA. 2012. Glyphosate-induced structural variations in Commelina erecta L. (Commelinaceae). Ecotoxicol. Environm. Safety 76: 135-142. https://doi.org/10.1016/j.ecoenv.2011.10.002
PANIGO, E. S., C. A. ALESSO, I. M. DELLAFERRERA, J. OLIVELLA & M. G. PERRETA. 2016. Morpho-architectural traits that allow the regeneration of Eustachys retusa (Poaceae) in systems with intensive glyphosate application. Revista Pl. Danin 34: 709-719. https://doi.org/10.1590/s0100-83582016340400011
PANIGO, E. S. & L. NISENSOHN. Commelina erecta L. En: FERNÁNDEZ; O. A., E. LEGUIZAMÓN, & H. A. ACCIARESI (ed.) “Malezas e Invasoras de la Argentina” Tomo III, pp. 181-189. Ediuns, Bahía Blanca.
PURICELLI, E. & D. FACCINI. 2005. Control de Eustachys retusa y Chloris barbata con glifosato. Soja en siembra directa. AAPRESID Septiembre: 112-123.
R DEVELOPMENT CORE TEAM. 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, Disponible en: http://www.R-project.org/.]
RAINERO, H. 2004. Avances en el control de malezas con tolerancia al glifosato. Bol. INTA- EEA Manfredi 12: 5-12.
SHIMIZU-SATO, S. & H. MORI. 2001. Control of outgrowth and dormancy in axillary buds. Pl. Physiol 127: 1405-1413. https://doi.org/10.1104/pp.010841
VESK, P. A. & M. WESTOBY. 2004. Sprouting ability across diverse disturbances and vegetation types worldwide. J. Ecol. 92: 310-320. https://doi.org/10.1111/j.0022-0477.2004.00871.x
VESK, P. A., D. I. WARTON & M. WESTOBY. 2004. Sprouting by semi-arid plants: testing a dichotomy and predictive traits. OIKOS 107: 72-89. https://doi.org/10.1111/j.0030-1299.2004.13122.x
WALDIE, T., A. HAYWARD & C. A. BEVERIDGE. 2010. Axillary bud outgrowth in herbaceous shoots: how do strigolactones fit into the picture? Pl. Molec. Biol. 73: 27-36. https://doi.org/10.1007/s11103-010-9599-2
Downloads
Publicado
Edição
Seção
Licença
Proporciona ACESSO ABERTO imediato e livre ao seu conteúdo sob o princípio de tornar a pesquisa livremente disponível ao público, o que promove uma maior troca de conhecimento global, permitindo que os autores mantenham seus direitos autorais sem restrições. 
Material publicado em Bol. Soc. Argent. Bot. é distribuído sob uma licença Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. 




