Morpho-anatomical features of the leaves and stems of Baccharis notosergila (Asteraceae) and their relationship with the environment and chemical control
DOI:
https://doi.org/10.31055/1851.2372.v56.n4.33519Palavras-chave:
aboveground system, adaptation features, environment, Salado river basin, stress tolerance.Resumo
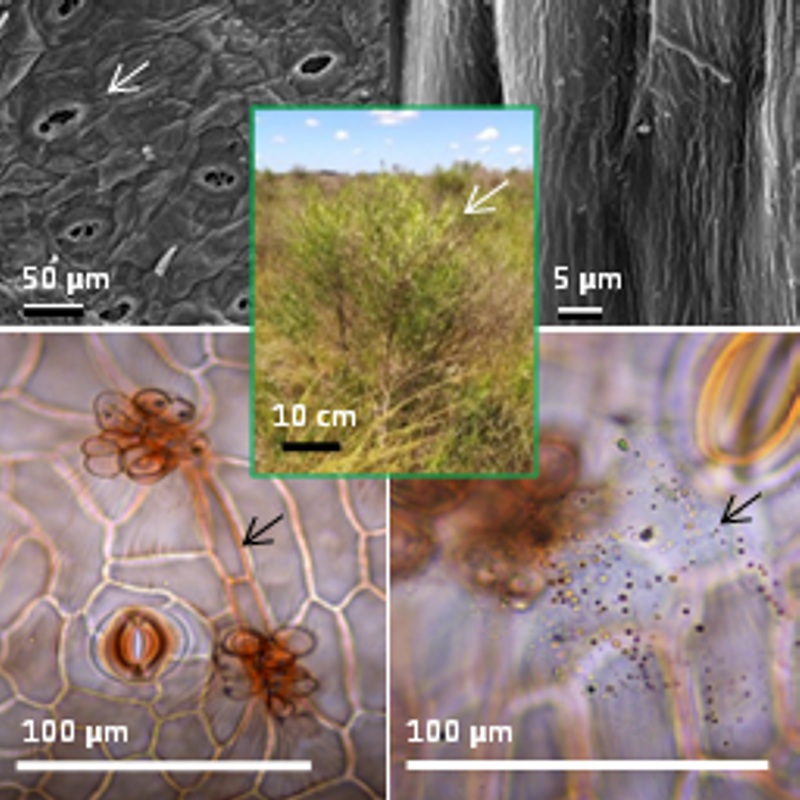
Background and aims: Baccharis notosergila is an aggressive weed inhabiting the Salado river basin, Buenos Aires province, Argentina. The aims of this work were: to analyze the morpho-anatomy and histochemistry of aerial vegetative organs in order to understand the adaptation strategies that ensure its survival, as well as to expand knowledge on traits determining resistance to the control methods applied.
M&M: The material collected was prepared and examined with conventional techniques of microscopy. Histochemical tests to identify starch, resins, polyphenols, and lipophilic substances were performed.
Results: The major features found were small and deciduous leaves; uniseriate epidermis with massive and striate cuticle; stomata at level or slightly above the other epidermal cells and glandular trichomes secreting oily substances; stomata on both surfaces and isobilateral mesophyll. Tannins, starch and lipophilic substances were identified in leaves and stems; polyphenols, resins and lipids in ducts, and calcium oxalate crystals in leaves, stems and capitate trichomes.
Conclusions: The aerial vegetative organs features of B. notosergila explain its tolerance to the unfavorable conditions of the Salado river basin area, as well as its high competitive ability over others species of the natural prairie. The reduced and deciduous leaves, the epidermal traits, and chemical substances found constitute a physical and chemical barrier reducing dehydration as well as the penetration of the herbicides applied for its control. Botanical knowledge of B. notosergila is the basis for the design and development of new and appropriate management methods for this species.
Referências
APÓSTOLO, N. M. 2005. Caracteres anatómicos de la vegetación costera del Río Salado (Noroeste de la provincia de Buenos Aires, Argentina). Bol. Soc. Argent. Bot. 40: 215-227.
APPEZZATO-DA-GLÓRIA, G. & G. CURY. 2011. Morpho-anatomical features of underground systems in six Asteraceae species from the Brazilian Cerrado.
An. Acad. Bras. Ciênc. 83: 981-991.
ARAMBARRI, A. M., M. C. NOVOA, N. D. BAYÓN, M. P. HERNÁNDEZ, M. N. COLARES & C. MONTI. 2011. Ecoanatomía foliar de árboles y arbustos de los Distritos Chaqueños Occidental y Serrano (Argentina). Bol. Soc. Argent. Bot. 46: 251-270.
ARIZA ESPINAR, L. 1973. Las especies de Baccharis (Compositae) de Argentina Central. Bol. Acad. Nac. Ci. 50: 1-305.
ASH, A., B. ELLIS, L. J. HICKEY, K. JOHNSON, P. WILF & S. WING. 1999. Manual of leaf architecture- morphological description and categorization of dicotyledonous and ner-veined monocotyledonous angiosperms by Leaf architecture working group. Smithsonian Institution, Washington, D. C.
BARTHLOTT, W., C. NEINHUIS, D. CUTLER, F. DITSCH, I MEUSEL, I. THEISEN & H. WILHELM. 1998. Classification and terminology of plant epicuticular waxes. Bot. J. Linn. Soc. 126: 237-260. https://doi.org/10.1111/j.1095-8339.1998.tb02529.x
BARRETO I. F., J. PADILHA DE PAULA, P. V. FARAGO, M. R. DUARTE & J. M. BUDEL. 2015. Pharmacobotanical study of the leaves and stems of Baccharis ochracea Spreng. for quality control. Lat. Am. J. Pharm. 34: 1497-1502.
BOBEK, V. B., V. P. DE ALMEIDA, C. B. PEREIRA, G. HEIDEN, M. R. DUARTE, J. M. BUDEL & T. NAKASHIMA. 2015. Comparative pharmabotanical analysis of Baccharis caprariifolia DC. and B. erioclada DC. From Campos Gerais, Paraná, Southern Brazil. Lat. Amer. J. Pharm. 34: 1396-1402.
BOBEK, V. B., G. HEIDEN, C. FREITAS DE OLIVEIRA, V. PAES DE ALMEIDA, J. PADILHA DE PAULA, P. V. FARAGO, T. NAKASHIMA & J. M. BUDEL. 2016. Comparative analytical micrographs of “vassouras” (Baccharis, Asteraceae). Rev. Bras. Farmacogn. 26: 665-672. https://dx.doi.org/10.1016/j.bjp.2016.05.001
BOLLER, T. 1989. Primary signals and second messengers in the reaction of plants to pathogens. In BOSS, W. F. & D. J. MORRÉ (eds.), Second Messengers in Plant Growth and Development, pp. 227-255. Alan R. Liss, New York.
BUDEL, J. M., M. R. DUARTE, C. A. M. SANTOS & L. M. CUNHA. 2003. Macro and microscopical identification of four species of Baccharis from Trimera group. Rev. Bras. Farmacogn. 13: 42-43. http://dx.doi.org/10.1590/S0102-695X2003000400014
BUDEL, J. M., M. R. DUARTE, C. A. M. SANTOS. 2004. Stem morpho-anatomy of Baccharis cylindrica (Less.) DC. (Asteraceae). Braz. J. Pharm. Sci. 40: 93-99. http://dx.doi.org/10.1590/S1516-93322004000100014
BUDEL, J. M. & M. R. DUARTE. 2007. Caracteres morfoanatômicos de partes vegetativas aéreas de Baccharis coridifolia DC. (Asteraceae-Astereae). Lat. Amer. J. Pharm. 26: 723-731.
BUDEL, J. M. & M. R. DUARTE. 2008. Estudo farmacobotânico de folha e caule de Baccharis uncinella DC., Asteraceae. Lat. Amer. J. Pharm. 27: 740-760.
BUDEL, J. M. & M. R. DUARTE. 2010. Macro and microscopic characters of the aerial vegetative organs of carqueja: Baccharis usterii Heering. Braz. Arch. Biol. Technol. 53: 123-131. https://doi.org/10.1590/S1516-89132010000100016
BUDEL, J. M., M. R. DUARTE, P. M. DÖLLBOSCARDIN, P. V. FARAGO, N. I.
MATZENBACHER, A. SARTORATTO & B. H. L. N. SALES MAIA. 2012. Composition of essential oils and secretory structures of Baccharis anomala, B. megapotamica and B. ochracea. J. Essent. Oil Res. 24: 19-24.
https://doi.org/10.1080/10412905.2012.645634
BUDEL, J. M., J. PADILHA DE PAULA, V. L. PEREIRA DOS SANTOS, C. R. CAVICHIOLO FRANCO, P. V. FARAGO & M. R. DUARTE. 2015. Pharmacobotanical study of Baccharis pentaptera. Rev. Bras. Farmacogn. 25: 314-319. http://dx.doi.org/10.1016/j.bjp.2015.07.007
BUDEL, J. M., V. RAMAN, L. M. MONTEIRO, V. P. ALMEIDA, V. B. BOBEK, G. HEIDEN, I. J. M. TAKEDA & I. A. KHAN. 2018. Foliar anatomy and microscopy of six Brazilian species of Baccharis (Asteraceae). Microsc. Res. Tech. 1-11. https://doi.org/10.1002/jemt.23045.
CARBONE, A.V. 2015. Caracterización morfo-anatómica de dos poblaciones de Gomphrena perennis L. y su posible relación con la sensibilidad al herbicida glifosato. Tesis de Maestría Protección Vegetal. Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata. Repositorio Institucional de la UNLP. Disponible en: http://sedici.unlp.edu.ar/bitstream/handle/10915/48707/Documento_completo.pdf-
PDFA.pdf?sequence=3&isAllowed=y
CARBONE, A. V., F. E. FERNÁNDEZ, M. P. HERNÁNDEZ & A. M. ARAMBARRI. 2019. Morphoanatomy, histochemistry and crystals of the underground system of Baccharis notosergila (Asteraceae). Bol. Soc. Argent. Bot. 54: 519-532. http://dx.doi.org/10.31055/1851.2372.v54.n4.24930
COBOS, M. I., J. L. RODRÍGUEZ, M. M. OLIVA, M. DEMO, S. M. FAILLACI & J. A. ZYGADLO. 2001. Composition and antimicrobial activity of the essential oil of Baccharis notosergila. Planta Med 67: 84-86. https://doi.org/10.1055/s-2001-10633
CORTADI, A., O. DI SAPIO, J. Mc CARGO, A. SCANDIZZI, S. GATTUSO & M. GATTUSO. 1999. Anatomical studies of Baccharis articulata, Baccharis crispa and Baccharis trímera, “carquejas” used in folk medicine. Pharm. Biol. 37: 357-365.
https://doi.org/10.1076/phbi.37.5.357.6054
COSA, M. T. & N. DOTTORI. 2010. Adaptaciones anatómicas de plantas medicinales a la diversidad de ambientes, 73 pp. Curso de actualización Profesional, X Simposio Argentino y XIII Simposio Latinoamericano de Farmacobotánica, Córdoba.
COSA, M. T., N. DOTTORI, L. STIEFKENS, M. HADID, M. MATESEVACH, N. DELBÓN, P. WIEMER, S. MACHADO, V. CABRERA, C. COSTA, A. PÉREZ & A. TRENCHI. 2014. Aplicación de técnicas de histología vegetal a la resolución de diversos problemas. Laboratorio de Morfología Vegetal, Universidad Nacional de Córdoba, Argentina.
DALL´ARMELLINA, A. A. & R. L. ZIMDAHL. 1989. Effect of watering frequency, drought, and glyphosate on growth of field bindweed (Convolvulus arvensis). Weed Sci. 37: 314- 318. https://www.jstor.org/stable/4044715
D’AMBROGIO, A. 1986. Manual de técnicas en histología vegetal. Hemisferio Sur, Buenos Aires, Argentina.
DELBÓN, N., M. T. COSA & G. BERNARDELLO. 2012. Exomorfología y anatomía de órganos vegetativos aéreos en especies de Flourensia DC. (Asteraceae) con importancia fitoquímica. Acta Bot. Brasil. 26: 2-10. https://doi.org/10.1590/S0102-33062012000100002
DEL VALLE, J. C., M. L. BUIDE, J. B. WHITTALL, F. VALLADARES & E. NARBONA. 2020. UV radiation increases phenolic compound protection but decreases reproduction in Silene littorea. PLoS One 15: e0231611. https://doi.org/10.13371/journal.pone.0231611.eCollection2020
DIAS, M. P., R. M. NOZARI & E. R. SANTARÉM. 2017. Herbicidal activity of natural compounds from Baccharis spp. On the germination and seedlings growth of Lactuca sativa and Bidens pilosa. Allelopathy J 42: 21-36. https://doi.org/10.26651/2017-42-1-1103
EBEL, J. 1986. Phytoalexin synthesis: The biochemical analysis of the induction process. Ann. Rev. Phytopathol. 24: 235-264. https://doi.org/10.1146/annurev.py.24.090186.001315
EHLERINGER, J. R. & H. A. MOONEY. 1978. Leaf hairs: effects on physiological activity and adaptive value to a desert shrub. Oecologia 37: 183-200. https://doi.org/10.1007/BF00344990
FAHN, A. & D. CUTLER. 1992. Xerophytes, pp. 1-143 Handbuchder Pflanzenantomie 13, 3. Gebruder Borntraeger, Berlín.
FRANCESCHI, V. R. & P. A. NAKATA. 2005. Calcium oxalate in plants: formation and function. Annu. Rev. Plant Biol. 56: 41-71.
https://doi.org/10.1146/annurev.arplant.56.032604.144106
FRANKLIN, G. L. 1945. Preparation of thin sections of synthetic resins and wood-resin composites, and a new macerating method for wood. Nature 155: 51.
https://doi.org/10.1038/155051a0
FREIRE, S. E., A.M. ARAMBARRI, N. D. BAYÓN, G. SANCHO, E. URTUBEY, C. MONTI, M. C. NOVOA & M. N. COLARES. 2005. Epidermal characteristics of toxic plants for cattle from the Salado river basin (Buenos Aires, Argentina). Bol. Soc. Argent. Bot. 40: 241-281.
FREIRE, S. E., E. URTUBEY & D. A. GIULIANO. 2007. Epidermal characters of Baccharis (Asteraceae) species used in traditional medicine. Caldasia 29: 23-38. https://dx.doi.org/10.15446/caldasia
GARCIA, M., D. JÁUREGUI & E. MEDINA. 2008. Adaptaciones anatómicas foliares en especies de angiospermas que crecen en la zona costera del Estado Falcón (Venezuela). Acta Bot.Venez. 31: 291-306.
GIDDA, S. K., S. PARK, M. PYC, O. YURCHENKO, Y. CAI, P. WU, D. W. ANDREWS, K. D. CHAPMAN, J. M. DYER & R. T. MULLEN. 2016. Lipid droplet-associated proteins (LDAPs) are required for the dynamic regulation of neutral lipid compartmentation in plant cells. Plant Physiol. 170(4): 2052-71. https://doi.org/10.1104/pp.15.01977.Epub 2016 Feb 19
GIULIANO, D. A. & A. PLOS. 2014. Baccharis. En: ZULOAGA, F. O., M. J. BELGRANO & A. M. ANTON (eds.). Flora Argentina: flora vascular de la República Argentina, Dicotyledoneae, Asteraceae, 7: 43-123. Estudio Sigma S. R. L., Buenos Aires.
GLAS, J. J., B. C. J. SCHIMMEL, J. M. ALBA, R. ESCOBAR-BRAVO, R. C. SCHUURINK & M. R. KANT. 2012. Plant glandular trichomes as targets for breeding of engineering of resistance to herbivores. Int. J. Mol. Sci. 13: 17077-17103. https://doi.org/10.3390/ijms131217077
GONZÁLEZ, A. M. 2018. Image J: una herramienta indispensable para medir el mundo biológico. Sociedad Argentina de Botánica. Folium (Relatos botánicos) 1: 6-17.
GURR, E. 1971. Synthetic dyes in biology, medicine and chemistry. Academic Press, London.
HABERLANDT, G. 1928. Physiological plant anatomy. Macmillan, London.
HASSANPOUR S., N. MAHERI-SIS, B. ESHRATKHAH & F. B. MEHMANDAR. 2011. Plants and secondary metabolites (Tannins): a review. Int. J. Forest, Soil and Erosion 1: 47-53. Available at: www.ijfse.com. [Accessed: 15 June 2020].
HAYASHI, A. H. & B. APPEZZATO-DA-GLÓRIA. 2007. Anatomy of the underground system in Vernonia grandiflora Less. and V. brevifolia Less. (Asteraceae). Braz. Arch. Biol. Technol. 50: 979-988. http://dx.doi.org/10.1590/S1516-89132007000700009
HEIDEN, G., J. R.VIEIRA IGANCI & L. MACIAS. 2009. Baccharis sect. Caulopterae (Asteraceae, Astereae) no Rio Grande do Sul, Brasil. Rodriguesia 60: 943-983.
DOI: 10.1590/2175-7860200960411
HEIDEN, G., A. ANTONELLI & J. R. PIRANI. 2019. A novel phylogenetic infrageneric classification of Baccharis (Asteraceae: Astereae), a highly diversified American genus. Taxon 68: 1048-1081.
https://doi.org/10.1002/tax.12128
INSTITUTO DE BOTÁNICA DARWINION.
Available at: http://www2.darwin.edu.ar/floraargentina//fa.htm. (Accessed: 15 June 2020).
JASINSKI, V. C. G., R. Z. da SILVA, R. PONTAROLO, J. M. BUDEL & F. R. CAMPOS. 2014. Morphoanatomical characteristics of Baccharis glaziovii in support of its pharmacobotany. Rev. Bras. Farmacogn. 24: 609-616. https://doi.org/10.1016/j.bjp.2014.11.003
JOHANSEN, D. A. 1940. Plant microtechnique. McGraw-Hill Book Company, New York.
JOHNSON, H. B. 1975. Plant pubescence: an ecological perspective. Bot. Rev. 41: 233- 258. https://doi.org/10-1007/BF02860838
JOHNSON, M. A. & R. CROTEAU. 1987. Biochemistry of conifer resistance to bark beetles and their fungal symbionts. In: Fuller, G. & W. D. Nes (eds.). Ecology and Metabolism of Plant Lipids, pp. 76-92. American Chemical Society, Washington, D.C.
LERSTEN, N. R. & J. D. CURTIS. 1985. Distribution and anatomy of hydathodes in Asteraceae. Int J Plant Sci 146: 106-114.
LERSTEN, N. R., A. R. CZLAPINSKI, J. D. CURTIS, R. FRECKMANN & H. T. HORNER. 2006. Oil bodies in leaf mesophyll cells of angiosperms: overview and a selected survey. Am J Bot 93: 1731-1739.
https://doi.org/10.3732/ajb.93.12.1731
LEVIN, D. 1973. The role of trichomes in plant defense. Q Rev Biol 48: 3-15.
LIESENFELD, V., P. GENTZ, E. M. DE FREITAS & S. MARTINS. 2019. Leaf morphology and anatomy of Asteraceae of the Pampas biome (sand-fields). FLORA 258, Article 151418. https://doi.org/10.1016/j.flora.2019.151418
LUQUE, R., H. C. SOUSA & J. E. KRAUS. 1996. Métodos de coloração de Roeser (1972) –modificadoe Kropp (1972) visando a substituição do azul de astra por azul de alcião 8 GS ou 8GX/Staining methods of modified Roeser (1972) and Kropp (1972), aiming at substituting the astra blue by alcian blue 8GS or 8GX. Acta Bot. Bras. 10: 199 - 212. http://dx.doi.org/10.1590/S0102-33061996000200001
MERIDA, T., J. SCHÖNHERR & H. W. SCHMIDT. 1981. Fine structure of plant cuticles in relation to water permeability: the fine structure of the cuticle of Clivia miniata reg. leaves. Planta 152: 259-267. https://doi.10.1007/BF00385154
METCALFE, C. R. & L. CHALK. 1950. Anatomy of the Dicotyledons. Clarendon Press, Oxford.
METCALFE, C. R. & L. CHALK. 1988. Anatomy of the Dicotyledons. Systematic anatomy of the leaf and stem. Clarendon Press, Oxford.
MINTEGUIAGA, M. 2019. Fitoquímica de Baccharis spp. L. (Asteraceae): metabolitos secundarios, semi-síntesis y bioactividad, pp. 577. Tesis presentada para aspirar al título de Doctor en Química. Cátedra de Farmacognosia y Productos Naturales, Laboratorio de Biotecnología de Aromas, Facultad de Química, Universidad de la República (UdelaR), Montevideo, Uruguay.
MOLANO-FLORES, B. 2001. Herbivory and calcium concentrations affect calcium oxalate crystal formation in leaves of Sida (Malvaceae). Ann. Bot. 88: 387-391. https://doi.org/10.1006/anbo.2001.1492
MOTT, K. A., C. A. GIBSON & J. W. O’LEARY. 1982. The adaptive significance of amphistomatic leaves. Plant Cell Environ 5: 455-460.
https://doi.org/10.1111/1365-3040.ep11611750
MOTT, K. A. & O. MICHAELSON. 1991. Amphistomaty as an adaptation to high light intensity in Ambrosia cordifolia (Compositae). Amer J Bot 78: 76-79. https://doi.org/10.1002/j.1537-2197.1991.tb12573.x
MÜLLER, J. 2013. World checklist of Baccharis L. (Compositae–Astereae), version 2013-09-03, pp. 170. Herbarium Haussknecht, Friedrich-Schiller-Universität, Jena.
NAKATA, P. A. 2003. Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Science 164: 901-909.
https://doi.org/10.1016/S0168-9452(03)00120-1
NUGHES, L., M. COLARES, M. HERNÁNDEZ & A. ARAMBARRI. 2013. Morfo-anatomía de las hojas de Celtis ehrenbergiana (Celtidaceae) desarrolladas bajo condiciones naturales de sol y sombra. Bonplandia 22: 47-58.
http://dx.doi.org/10.30972/bon.2221245
O`BRIEN, T. P., N. FEDER & M. E. MC CULLY. 1964. Polychromatic stained of plant cell walls by toluidine blue O. Protoplasma 59: 368-373. https://doi.org/10.1007/BF01248568
OLIVEIRA, V. C. & E. M. BASTOS. 1998. Aspectos morfo-anatômicos da folha de Baccharis dracunculifolia DC. (Asteraceae) visando a identificação da origem botánica da própolis. Acta Bot. Bras. 12: 431-439. https://doi.org/10.1590/S0102-33061998000400012
OLIVEIRA, A.M.A., V.L.P. SANTOS, C.R.C. FRANCO, P.V. FARAGO, M.R. DUARTE & J.M BUDEL. 2011. Comparative morpho-anatomy study of Baccharis curitybensis Heering ex Malme and Baccharis spicata (Lam.) Baill. Lat. Am. J. Pharm. 30: 1560-1566.
ORNELLAS, T., G. HEIDEN, B. NUNES DE LUNA & C. FRANCA BARROS. 2019. Comparative leaf anatomy of Baccharis (Asteraceae) from high-altitude grassland in Brazil: taxonomic and ecological implications. Botany 97: 615-626. NRC Research Press. https://dx.doi.org/10.1139/cjb-2019-0035
PARKHURST, D. F. 1978. The adaptative significance of stomatal occurrence on one or both surfaces of leaves. J. Ecol. 66: 367-383. https://doi.org/10.2307/2259142
PEREIRA, C.B., P.V. FARAGO, J.M. BUDEL, J.P. DE PAULA, D.G. FOLQUITTO & O. G. MIGUEL & M. D. MIGUEL. 2014. A new contribution to the pharmacognostic study of carquejas: Baccharis milleflora DC., Asteraceae. Lat. Am. J. Pharm. 33: 841-847.
PÉREZ, A. & V. H. TOMASI. 2002. Tinción con azul brillante de cresilo en secciones vegetales con parafina. Bol. Soc. Argent. Bot. 37: 211-215.
PETENATTI, E. M., M. E. PETENATTI, D. A. CIFUENTE, J. C. GIANELLO, O. S. GIORDANO, C. E. TONN & L. A. DEL VITTO. 2007. Medicamentos herbarios en el Centro-Oeste Argentino. VI. Caracterización y control de calidad de dos especies de “carquejas”: Baccharis sagittalis y B. triangularis (Asteraceae). Lat. Am. J. Pharm. 26: 201-208.
PIHAKASKI, K., S. PIHAKASKI, P. KARUNEN & P. KALLIO. 1987. Seasonal changes in leaf lipids of Diapensia japponica, with special reference to storage lipid bodies. Nord. J. Bot. 7: 281-292. https://doi.org/10.1111/j.1756-1051.1987.tb00945.x
RAMAYYA, N. 1962. Studies on the trichomes of some Compositae I. General structure. Bull. Bot. Surv. India 4: 177-188.
RICE, E.L. 1984. Allelopathy, Second edition. Academic Press, New York.
RODRIGUEZ, A & E. JACOBO. 2012. Manejo de pastizales naturales para una ganadería sustentable en la Pampa Deprimida. Buenas prácticas para una ganadería sustentable de pastizal. Kit de extensión para las pampas y campos. Editorial FAUBA, Buenos Aires.
ROTH, I. 1984. Stratification of tropical forests as seen in leaf structure. In Encyclopedia of plant anatomy, Gebrüder Borntraeger, Berlin.
RUZIN, S. E. 1999. Plant microtechnique and microscopy. University Press, Oxford.
RYAN, C.A. 1987. Oligosaccharide signaling in plants. Annu Rev Cell Biol 3: 295-317. https://doi.org/10.1146/annurev.cb.03.110187.001455
SANTIER, S. & A. CHAMEL. 1992. Penetration of glyphosate and diuron into and through isolated plant cuticles. Weed Res. 32: 337-347. https://doi.org/10.1111/j.1365-3180.1992.tb01894.x
SHIMADA, T. L., Y. TAKANO, T. SHIMADA, M. FUJIWARA, Y. FUKAO, M. MORI, Y.OKAZAKI, K. SAITO, R. SASAKI, K. AOKI & I. HARA-NISHIMURA. 2014. Leaf oil body functions as a subcellular factory for the production of a phytoalexin in Arabidopsis. Plant Physiol. 164: 105-118. https://doi.org/10.1104/pp.113.230185
SHIMADA, T. L., M. HAYASHI & I. HARA-NISHIMURA. 2018. Membrane dinamics and multiple functions of oil bodies in seeds and leaves. Plant Physiol. 176(1): 199-207.
https://doi.org/10.1104/pp.17.01522
SIONE, S. M., R. A. SABATTINI, S. G. LEDESMA, A. F. DORSCH & C. FORTINI. 2006. Caracterización florística y structural del estrato arbustivo de un monte en pastoreo (Las Garzas, Entre Ríos). Rev. Cient. Agropecu. 10: 59-67.
SMILJANIC, K. B. A. 1969. Anatomia foliar de espécies de Asteraceae de um afloramento rochoso no Parque Estadual da Serra do Brigadeiro (MG), Viçosa, pp.79. Tesis Magister Science. Universidade Federal de Viçosa, Brazil.
SOUZA, C. A. de, P. V. FARAGO, M. R. DUARTE & J. M. BUDEL. 2011. Pharmacological study of Baccharis singularis (Vell.) G. M. Barroso, Asteraceae. Lat. Am. J. Pharm. 30: 311-317.
STENGLEIN, S., A. M. ARAMBARRI, M. C. MENÉNDEZ SEVILLANO & P. A. BALATTI. 2005. Leaf epidermal characters related with plant’s passive resistance to pathogens vary among accessions of wild beans Phaseolus vulgaris var. aborigineus (Leguminosae-Phaseoleae). Flora 200: 285-295. https://doi.org/10.1016/j.flora.2005.01.004
STONE, B. A. 1989. Cell walls in plant-microorganism associations. Aust. J. Plant Physiol. 16: 5-17. https://doi.org/10.1071/PP9890005
TAPIA-TORRES, N. A., C. de la PAZ-PÉREZ-OLVERA, A. ROMÁN GUERRERO, A. QUINTANAR-ISAÍAS, E. GARCÍA-MÁRQUEZ & F. CRUZ-SOSA. 2014. Histoquímica, contenido de fenoles totales y actividad antioxidante de hoja y de madera de Litsea glaucescens Kunth (Lauraceae). Madera y Bosques 20: 125-137.
TOSORATTO, N., M. T. COSA & N. DELBÓN. 2016. Morfoanatomía e histoquímica de cuatro Asteraceae nativas del Bosque Chaqueño Serrano (Córdoba, Argentina). Bol. Soc. Argent. Bot. 51: 613-622.
URDAMPILLETA, J. I. 2019. Métodos de control poblacional de Baccharis notosergila Griseb.: maleza arbustiva de alta incidencia en la zona de la Pampa Deprimida. Tesis de grado, modalidad: intervención profesional, Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata. Repositorio Institucional de la UNLP. Disponible en:
http://sedici.unlp.edu.ar/handle/10915/74210
VOLK, G. M., V. J. LYNCH-HOLM, T. KOSTMAN, L. J. GOSS & V. FRANCESCHI. 2002. The role of druse and raphide calcium oxalate crystals in tissue calcium regulation in Pistia stratioides leaves. Plant Biology 4: 34-45.
https://doi.org/10.1055/s2002-20434
WESTWOOD, J. H., C. N. YERKES, F. P. DEGENNARO & S. C. WELLER. 1997. Absorption and translocation of glyphosate in tolerant and susceptible biotypes of field bindweed (Convolvulus arvensis). Weed Sci. 45: 658-663.
https://doi.org/10.1017/S0043174500093292
ZARLAVSKY, G. E. (ed.). 2014. Histología vegetal: Técnicas simples y complejas. Sociedad Argentina de Botánica, Buenos Aires.
ZULOAGA, F. O., M. J. BELGRANO & C. A. ZANOTTI. 2019. Actualización del Catálogo de las plantas vasculares del Cono Sur. Darwiniana, nueva serie 7: 208-278. https://doi.org/10.14522/darwiniana.2019.72.861
Downloads
Publicado
Edição
Seção
Licença
Copyright (c) 2021 Alejandra V. Carbone, Federico E. Fernández, Marcelo P. Hernández, Santiago M. Martínez Alonso, Ana Maria Arambarri

Este trabalho está licenciado sob uma licença Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Proporciona ACESSO ABERTO imediato e livre ao seu conteúdo sob o princípio de tornar a pesquisa livremente disponível ao público, o que promove uma maior troca de conhecimento global, permitindo que os autores mantenham seus direitos autorais sem restrições. 
Material publicado em Bol. Soc. Argent. Bot. é distribuído sob uma licença Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. 




