Spore viability of fern species of the genera Amauropelta, Blechnum, and Physematium from central Argentina after low temperature storage
DOI:
https://doi.org/10.31055/1851.2372.v59.n3.44658Keywords:
Amauropelta, Blechnum, ex-situ conservation, fern, freezing, Physematium, spore longevityAbstract
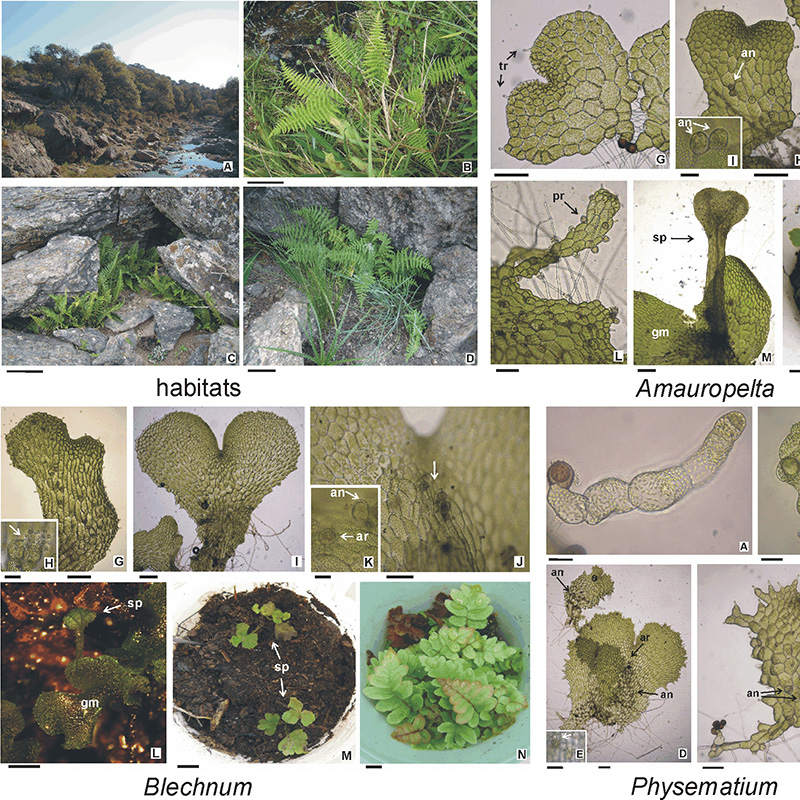
Background and aims: Ferns are sensitive to environmental changes, inhabiting ecosystems susceptible to degradation. Spore banks and in vitro spore culture are useful tools employed in their conservation. The objective was to test the ability of the spores to germinate after being stored in a freezer and to develop gametophytes and sporophytes, in native fern species of genera Amauropelta, Blechnum and Physematium.
M&M: Spores of A. argentina, B. auriculatum and P. montevidensis were stored at -20 ºC in dry conditions for 6 and 12 months. They were sown in vitro in Dyer liquid medium and incubated in a growth chamber. The means of germination percentages were calculated and a one-way Student’s t-test was employed. Gametophyte and sporophyte development was registered under light and stereoscopic microscopes.
Results: Depending on the species, statistical differences were recorded in germination percentages (viability) between both storage periods. The spores of A. argentina kept in freezer for 12 months completely lost viability. In B. auriculatum, viability decreased over time, and in P. montevidensis it remained constant. Gametophytes and sporophytes developed in all cultures; except in A. argentina spores, with 12 months in freezer.
Conclusions: The viability of the spores under dry storage at low temperature could be conditioned by the ecological requirements of the species. Protocols used for spore storage and culture allowed obtaining gametophytes and sporophytes in trials.
References
ARAGÓN, C. F. & E. PANGUA. 2004. Spore viability under different storage conditions in four rupicolous Asplenium L. taxa. Am. Fern. J. 94: 28-38.
ARANA, M., M. PONCE & N. VISCHI. 2004. Sinopsis de los helechos y grupos relacionados (Pteridophyta) de la provincia de Córdoba, Argentina. Bol. Soc. Argent. Bot. 39: 89-114.
ARANA, M. D., J. J. MORRONE, M. M. PONCE. & A. J. OGGERO. 2013. Patrones biogeográficos de los helechos de las sierras de Córdoba (Argentina) y sus implicancias en la conservación. Gayana Bot. 70: 357-376. http://dx.doi.org/10.4067/S0717-66432013000200013
ARANA, M. D., E. NATALE, A. OGGERO, N. FERRETI, …, & J. J. MORRONE. 2021. Esquema biogeográfico de la República Argentina. Opera Lilloana 56: 1- 240.
ARCAND, N. N. & T. A. RANKER. 2008. Conservation biology. En: RANKER, T. A. & C. H. HAUFLER (eds.), Biology and evolution of ferns and lycophytes, pp. 257-283. Cambridge University Press, Cambridge.
ARGARAÑAZ, J. P., A. M. CINGOLANI, L. M. BELLIS & M. A. GIORGIS. 2020. Fire incidence along an elevation gradient in the mountains of central Argentina. Ecol. Austral 30:268-281. https://doi.org/10.25260/EA.20.30.2.0.1054
BALLESTEROS, D., E. ESTRELLES & A. M. IBARS. 2006. Responses of Pteridophyte spores to ultrafreezing temperatures for long term conservation ingermplasm banks. Fern Gaz. 17: 293-302.
BALLESTEROS, D. 2010. Conservation of Fern Spores. En: FERNÁNDEZ, H., A. KUMAR & M. A. REVILLA (eds.), Working with ferns: issues and applications, pp. 165-172. Springer, New York.
BALLESTEROS, D., E. ESTRELLES, C. WALTERS & A. M. IBARS. 2012. Effects of temperature and desiccation on ex situ conservation of nongreen fern spores. AJB 99: 721-729.
BALLESTEROS, D. & V. C. PENCE. 2018. Fern conservation: spore, gametophyte, and sporophyte ex situ storage, in vitro culture, and cryopreservation. En: FERNANDEZ, H. (ed.) Current advances in fern research, pp. 227-249. Springer, New York.
BREMAN, E., BALLESTEROS, D., CASTILLO-LORENZO, E., COCKEL, …, & T. ULIAN. 2021. Plant diversity conservation challenges and prospects-the perspective of botanic gardens and the Millennium Seed Bank. Plants 10: 2371. https://doi.org/10.3390/plants10112371
DELLA, A. P. & D. G. FALKENBERG. 2019. Pteridophytes as ecological indicators: an overview. Hoehnea 46: e522018. https://doi.org/10.1590/2236-8906-52/2018
DURÁN, M. L. 1997. Estudios morfológicos, taxonómicos y biosistemáticos en el género Blechnum (Blechnaceae-Pteridophyta). Tesis Doctoral, Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de Córdoba, Argentina.
DYER, A. F. 1979. The Experimental Biology of Ferns. Trans. Bot. Soc. Edinburgh 43: 75-90.
DYER, A. F. 1994. Natural soil spore banks-can they be used to retrieve lost ferns? Biodivers. Conserv. 3:160-175.
FILIPIN, E. P., E. C. SCHMIDT, J. B. BARUFI, Z. L. BOUZON & A. M. RANDI. 2016. The Gametophyte of Pleopeltis lepidopteris (Langsd. & Fisch.) de la Sota (Polypodiaceae), a fern from restinga, after spore cryopreservation: morphological, ultrastructural, and physiological analyses. IJPS 177: 294-303.
GABRIEL Y GALÁN, J. M. & C. PRADA. 2010. Pteridophyte spores viability. En: HERNÁNDEZ, H., A. KUMAR & M. A. REVILLA (eds.), Working with ferns: issues and applications, pp. 193-205. Springer, New York.
GOSWAMI, H. K., K. SEN & R. MUKHOPADHYAY. 2016. Pteridophytes: evolutionary boon as medicinal plants. Plant Genet. Resour. 14: 328-355.
https://doi.org/10.1017/S1479262116000290
HUANG, Y. M., Y. H. CHANG & W. L. CHIOU. 2019. Edible ferns and lycophytes in Asia. Fern Gaz. 21:45-68.
IBARS A. M. & E. ESTRELLES. 2012. Recent developments in ex situ and in situ conservation of ferns. Fern Gaz. 19:67-86.
LEE, C. H. & S. L. SHIN. 2011. Functional activities of ferns. En: FERNÁNDEZ, H., A. KUMAR & M. A. REVILLA (eds.), Working with ferns: issues and applications, pp. 347-359. Springer, New York.
LIU, Y., W. WUJISGULENG & C. LONG. 2012. Food uses of ferns in China: a review. Acta Soc. Bot. Pol. 81: 263-270. https://doi.org/10.5586/asbp.2012.046
MANNAN, M., M. MARIDASS & B. VICTOR. 2008. A review on the potential uses of ferns. EBL 12: 281-285.
MARTINENCO, M. L., M. D. ARANA & M. L. LUNA. 2023. Morphogenesis of the gametophyte of Physematium montevidensis (Woodsiaceae). Rodriguésia 74: e00742023. https://doi.org/10.1590/2175-7860202374082
MEHLTRETER, K. 2010. Fern conservation. En: MEHLTRETER, K., L. R. WALKER, J. M. SHARPE (eds.), Fern Ecology, pp. 323-359. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9780511844898.010
MIKULA, A., K. JATA & J. J. RYBCZYNKSI. 2009. Cryopreservation strategies for Cyathea australis (R.Br.) Domin. CryoLetters 30: 429-439.
MIKUŁA, A., K. TOMICZAK, D. MAKOWSKI, M. NIEDZIELSKI & J. J. RYBCZYŃSKI. 2015. The effect of moisture content and temperature on spore aging in Osmunda regalis. APP 37: 1-11.
MORGAN, P., C. C. HARDY, T. W. SWETNAM, M. G. ROLLINS & D. G. LONG. 2001. Mapping fire regimes across time and space: understanding coarse and fine-scale patterns. IJWF 10: 329-342. https://doi.org/10.1071/WF01032
NAYAR, B. K. & S. KAUR. 1971. Gametophytes of homosporous ferns. Bot. Rev. 37: 295-396.
OGGERO, A. J. & M. D. ARANA. 2012. Inventario de la biodiversidad de plantas vasculares del sur de la zona serrana de Córdoba, Argentina. Hoehnea 39: 169-197. https://doi.org/10.1590/S2236-89062012000200002
PENCE, V. C. 2002. Cryopreservation and in vitro methods for ex situ conservation of Pteridophytes. Fern Gaz. 16: 362-369.
PENCE, V. C. 2008. Ex situ conservation of ferns and lycophytes-approaches and techniques. En: RANKER, T. A. & C. H. HAUFFLER (eds.), Biology and evolution of ferns and lycophytes, pp. 284-300. Cambridge University Press, Cambridge.
PENCE, V. C. 2015. Propagation and cryopreservation of Asplenium scolopendrium var. americanum, the American hart’s-tongue fern. Am. Fern J. 105: 211-225.
https://doi.org/10.1640/0002-8444-105.3.211
PENCE, V. C. 2018. Growth of fern gametophytes after 20 years of storage in liquid nitrogen. Fern Gaz. 20: 337-346.
QUINTANILLA, L. G., J. AMIGO, E. PANGUA & S. PAJARON. 2002. Effect of storage method on spore viability in five globally threatened fern species. Ann. Bot. 904: 461-467.
SHARPE, J. M., K. MEHLTRETER & L. R. WALKER. 2010. Ecological importance of ferns. En: MEHLTRETER, K., L. R. WALKER & J. M. SHARPE (eds.), Fern Ecology, pp. 1-21. Cambridge University Press, Cambridge.
SHEFFIELD, E. 1996. From pteridophyte spore to sporophyte in the natural environment. En: CAMUS, M. G. J. M. & R. JOHNS (eds.), Pteridology in perspective, pp. 541-549. Royal Botanic Gardens, Kew.
TAYLOR, S. & L. KUMAR. 2016. Global climate change impacts on pacific islands terrestrial biodiversity: a review. Trop. Conserv. Science 9: 203-223.
https://doi.org/10.1177/194008291600900111
TOMIZAC, K., D. MAKOWSKI, E. SLIWINSKA & A. MIKULA. 2023. The development of an in vitro propagation and conservation system for the endangered serpentine fern Asplenium cuneifolium. PCTOC 154: 161-175. https://doi.org/10.1007/s11240-023-02524-4
VARGAS, I. B. D. & A. DROSTE. 2014. In vitro propagation of Cyathea atrovirens (Cyatheaceae): spore storage and sterilization conditions. Rev. Biol. Trop. 62: 359-368.
WHELAN, R. J. 1995. The ecology of fire. Cambridge University Press, Cambridge.
Published
Issue
Section
License
Copyright (c) 2024 Maria L. Martinenco, Marcelo D. Arana, M. Lujan Luna

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Provides immediate and free OPEN ACCESS to its content under the principle of making research freely available to the public, which fosters a greater exchange of global knowledge, allowing authors to maintain their copyright without restrictions.
Material published in Bol. Soc. Argent. Bot. is distributed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International license.




