Estrategias fotosintéticas y morfo-anatómicas foliares de Ligustrum sinense (Oleaceae), una especie exótica invasora en el sotobosque de la selva de yungas.
DOI:
https://doi.org/10.31055/1851.2372.v58.n2.40335Palabras clave:
Anatomía foliar, bosque subtropical, eficiencia en uso de agua, fotosíntesis, invasión biológica, ligustrina, Noroeste ArgentinoResumen
Introducción y objetivos: Ligustrum sinense (ligustrina) es una reconocida especie exótica invasora. El objetivo de este trabajo fue estudiar los rasgos fisiológicos y anatómicos foliares de renovales de ligustrina que podrían otorgarle ventajas para su establecimiento en un ambiente de sotobosque de la selva de Yungas.
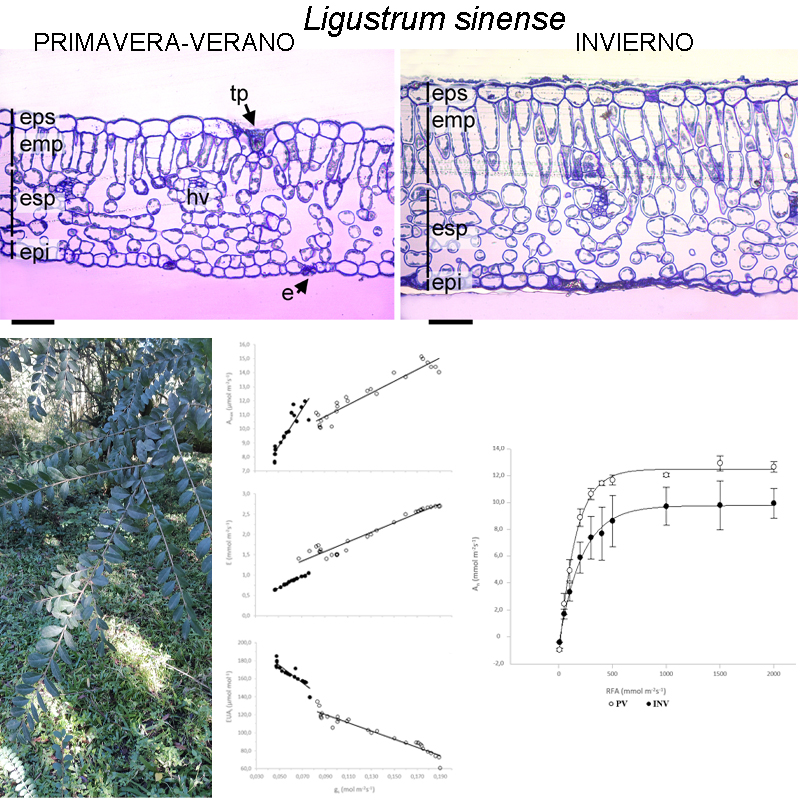
M&M: Este estudio se realizó en el Parque Nacional Aconquija (Tucumán, Argentina). Se evaluaron los rasgos morfo-anatómicas foliares y fisiológicos en un ambiente de sotobosque durante primavera-verano e invierno. Se analizaron las características anatómicas de la lámina foliar y variables fisiológicas relacionadas al intercambio de gases. Además, se calculó el área foliar específica, densidad de hojas, contenido de nitrógeno foliar y eficiencia fotosintética en el uso del nitrógeno foliar.
Resultados: Durante primavera-verano L. sinense muestra una mayor eficiencia fotosintética en el uso del nitrógeno foliar y una mayor asimilación fotosintética en condiciones saturantes. La eficiencia de carboxilación, eficiencia intrínseca del uso del agua y espesor de hoja fueron 26%, 34% y 41% mayores en invierno con relación a primavera-verano. Durante el invierno se registró un aumento en el espesor de la lámina lo que contribuiría a mejorar el intercambio de CO2 en época de condiciones subóptimas para la fotosíntesis.
Conclusiones: La ligustrina fotosintetiza todo el año y su éxito para establecerse en el sotobosque de la selva de Yungas podría explicarse en parte por las estrategias morfo-anatómicas y fisiológicas desplegadas.
Referencias
AINSWORTH, E. A., P. A. DAVEY, G. J. HYMUS, C. P. OSBORNE, … & S. P. LONG. 2003. Is stimulation of leaf photosynthesis by elevated carbon dioxide concentration maintained in the long term? A test with Lolium perenne grown for 10 years at two nitrogen fertilization levels under Free Air CO2 Enrichment (FACE). Plant Cell Environ. 26: 705-714. https://doi.org/10.1046/j.1365-3040.2003.01007.x
APN. 2016. Plan de Gestión Parque Nacional Campo de los Alisos [online]. Disponible en: https://sib.gob.ar/archivos/PG_PNCAlisos_julio_2016_comp.pdf [Acceso: 20 marzo 2023].
ARAGÓN, R. & M. GROOM. 2003. Invasion by Ligustrum lucidum (Oleaceae) in NW Argentina: Early stages characteristics in different habitat types. Rev. Biol. Trop. 51: 59-70.
ARANDA, I., M. PARDOS, J. PUERTOLAS, M. D. JIMENEZ & J. A. PARDOS. 2007. Water-use efficiency in cork oak (Quercus suber) is modified by the interaction of water and light availabilities. Tree Physiol. 27: 671-677.
https://doi.org/10.1093/treephys/27.5.671.
AWADA, T., K. RADOGLOU, M. N. FOTELLI & H. I. A. CONSTANTINIDOU. 2003. Ecophysiology of seedlings of three Mediterranean pine species in contrasting light regimes. Tree Physiol. 23: 33-41. https://doi.org/10.1093/treephys/23.1.33.
BARUCH, Z. & G. GOLDSTEIN. 1999. Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawaii. Oecologia 121: 183-192. https://doi.org/10.1007/s004420050920.
BATCHER, M. S. 2000. Element stewardship abstract for Ligustrum spp., privet. The Nature Conservancy, Arlington, Virginia [online]. Disponible en: https://www.invasive.org/weedcd/pdfs/tncweeds/ligu_sp.pdf. [Acceso: 20 marzo 2023].
BIANCHI, A. R. & C. E. YÁÑEZ. 1992. Las precipitaciones en el Noroeste Argentino. 2° Ed. INTA EEA Salta, Salta.
BROOKS, M. L., C. M. D’ANTONIO, D. M. RICHARDSON, J. B. GRACE, … & D. PYKE. 2004. Effects of invasive alien plants on fire regimes. BioScience 54: 677-688. https://doi.org/10.1641/0006-3568(2004)054[0677:EOIAPO]2.0.CO;2
BUSNELLI, J. 2009. Evolución histórica, situación actual y perspectivas futuras del riesgo de inundación en la cuenca del Río Gastona, Tucumán, Argentina. Tesis Doctoral. Universidad Nacional de Tucumán, Argentina.
CABRERA, A. 1976. Regiones Fitogeográficas Argentinas. Enciclopedia Argentina de Agricultura y Jardinería. 2° Ed. Editorial ACME SACI, Buenos Aires.
CASH, J. S., C. J. ANDERSON & W. D. GULSBY. 2020. The ecological effects of Chinese privet (Ligustrum sinense) invasion: a synthesis. Invasive Plant Sci. Manag. 13: 3-13. https://doi.org/10.1017/inp.2020.4
CAVALERI, M. A. & L. SACK. 2010. Comparative water use of native and invasive plants at multiple scales: a global meta-analysis. Ecology 91: 2705-2715. https://doi.org/10.1890/09-0582.1
CEBALLOS, S. J., Y. JIMÉNEZ & R. FERNÁNDEZ. 2020. Estructura de los bosques de Gleditsia triacanthos en función de la edad (valle de La Sala, Tucumán, Argentina). Ecol. Austral 30: 251-259. https://doi.org/10.25260/EA.20.30.2.0.1083
CRAINE, J. M. & P. B. REICH. 2005. Leaf‐level light compensation points in shade‐tolerant woody seedlings. New Phytol. 166: 710-713.
https://doi.org/10.1111/j.1469-8137.2005.01420.x
DIZEO DE STRITTMATTER, C. G. 1973. Nueva técnica de diafanización. Bol. Soc. Argent. Bot. 15: 126-129.
https://botanicaargentina.org.ar/wp-content/uploads/2018/09/126-129013.pdf
DONG, T., J. LI, Y. ZHANG, H. KORPELAINEN, … & C. LI. 2015. Partial shading of lateral branches affects growth, and foliage nitrogen- and water-use efficiencies in the conifer Cunninghamia lanceolata growing in a warm monsoon climate. Tree Physiol. 35: 632-643. https://doi.org/10.1093/treephys/tpv036
DRAKE, J. A., H. A. MOONEY, F. DI CASTRI, R. GROVES, F. KRUGER, M. REJMANEK & M. WILLIAMSON. 1989. Biological invasion. A global perspective. Scientific Committee on Problems of the Environment, International Council of Scientific Unions. Wiley, New York.
DURAND, L. Z. & G. GOLDSTEIN. 2001. Photosynthesis, photoinhibition, and nitrogen use efficiency in native and invasive tree ferns in Hawaii. Oecologia 126: 345-354. https://doi.org/10.1007/s004420000535
FERNÁNDEZ, R. D., S. J. CEBALLOS, R. ARAGÓN, A. MALIZIA, … & H. R. GRAU. 2020. A global review of Ligustrum lucidum (Oleaceae) invasion. Bot. Rev. 86: 93-118. https://doi.org/10.1007/s12229-020-09228-w
FLEXAS, J., J. GULÍAS, S. JONASSON, H. MEDRANO & M. MUS. 2001. Seasonal patterns and control of gas exchange in local populations of the Mediterranean evergreen shrub Pistacia lentiscus L. Acta Oecol. 22: 33-43.
https://doi.org/10.1016/S1146-609X(00)01099-7
FLEXAS, J., M. M. BARBOUR, O. BRENDEL, H. M. CABRERA, … & C. R. WARREN. 2012. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Science 193-194: 70-84. https://doi.org/10.1016/j.plantsci.2012.05.009.
FUNK, J. L., L. A. GLENWINKEL & L. SACK. 2013. Differential allocation to photosynthetic and non-photosynthetic nitrogen fractions among native and invasive species. PLoS ONE 8: e64502. https://doi.org/10.1371/journal.pone.0064502
GIORGIS, M. A., P. A. TECCO, A. M. CINGOLANI, D. RENISON, … & V. PAIARO. 2011. Factors associated with woody alien species distribution in a newly invaded mountain system of central Argentina. Biol. Invasions 13: 1423-1434. https://doi.org/10.1007/s10530-010-9900-y
GONZÁLEZ, J. A., S. E. BUEDO & F. E. PRADO. 2017. Caracterización fotosintética en plantas jóvenes y adultas de Alnus acuminata (“aliso del cerro”) en las Yungas (Tucumán, Argentina). Lilloa 54: 41-57.
https://www.lillo.org.ar/journals/index.php/lilloa/article/view/80
KARNOVSKY, M. J. 1965. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 27: 137-138.
KAUSHIK, P., P.K. PATI, M.L. KHAN & P.K. KHARE. 2022. Plant functional traits best explain invasive species’ performance within a dynamic ecosystem-A review. Trees, Forests and People. 100260. https://doi.org/10.1016/j.tfp.2022.100260
KIRSCHBAUM, M. U. 2011. Does enhanced photosynthesis enhance growth? Lessons learned from CO2 enrichment studies. Plant Physiol. 155: 117-124. https://doi.org/10.1104/pp.110.166819
LACORETZ, M.V., C. MALAVERT, N. MADANES, P. CRISTIANO & P.M. TOGNETTI. 2022. Seed dormancy and germination of native and invasive alien woody species of an endangered temperate forest in the Argentine Pampas. For. Ecol. Manag. 526: 120577. https://doi.org/10.1016/j.foreco.2022.120577
LAMBERS, H. & H. POORTER. 1992. Inherent variation in growth rate between higher plants: A search for physiological causes and ecological consequences. Adv. Ecol. Res. 23: 187-261. https://doi.org/10.1016/S0065-2504(08)60148-8
LARCHER, L., G. NOGUEIRA & M. R. BOEGER. 2015. Morphological plasticity and gas exchange of Ligustrum lucidum W.T. Aiton in distinct light conditions. Braz. Arch. Biol. Technol. 58: 877-885. https://doi.org/10.1590/S1516-89132015060439
LIANG, H-Z, F. ZHU, R-J WANG, X-H HUANG & J-J CHU. 2019. Photosystem II of Ligustrum lucidum in response to different levels of manganese exposure. Sci. Rep. 9: 12568. https://doi.org/10.1038/s41598-019-48735-8.
LICHSTEIN, J. W., H. R. GRAU & R. ARAGÓN. 2004. Recruitment limitation in secondary forests dominated by an exotic tree. J. Veg. Sci. 15: 721-728. https://doi.org/10.1111/j.1654-1103.2004.tb02314.x
LUNDGREN, M. R. & A. J. FLEMING. 2020. Cellular perspectives for improving mesophyll conductance. Plant J. 101: 845-857. https://doi.org/10.1111/tpj.14656
MACK, R. N., D. SIMBERLOFF, W. M. LONSDALE, H. EVANS, … & F. A. BAZZAZ. 2000. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 10: 689-710.
https://doi.org/10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2
MARENCO, R. A., J .F. DE C. GONCALVES & G. VIEIRA. 2001. Leaf gas exchange and carbohydrates in tropical trees differing in successional status in two light environments in central Amazonia. Tree Physiol. 21: 1311-1318. https://doi.org/10.1093/treephys/21.18.1311
MATZEK, V. 2011. Superior performance and nutrient-use efficiency of invasive plants over non-invasive congeners in a resource-limited environment. Biol. Invasions 13: 3005-3014. https://doi.org/10.1007/s10530-011-9985-y
MEDLYN, B. E., E. DREYER, D. ELLSWORTH, M. FORSTREUTER, … & D. LOUSTAU. 2002. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ. 25: 1167-1179. https://doi.org/10.1046/j.1365-3040.2002.00891.x
MILANOVIĆ, M., S. KNAPP, P. PYŠEK & I. KÜHN. 2020. Linking traits of invasive plants with ecosystem services and disservices. Ecosyst. Serv. 42: 101072. https://doi.org/10.1016/j.ecoser.2020.101072
MIYAZAWA, Y. & K. KIKUZAWA. 2005. Winter photosynthesis by saplings of evergreen broad‐leaved trees in a deciduous temperate forest. New Phytol. 165: 857-866. https://doi.org/10.1111/j.1469-8137.2004.01265.x
MO, L., J. CHEN, X. LOU, Q. XU, … & E. LIN. 2020. Colchicine-induced polyploidy in Rhododendron fortunei Lindl. Plants 9: 424.
https://doi.org/10.3390/plants9040424
MONTALDO, N. H. 1993. Dispersión por aves y éxito reproductivo de dos especies de Ligustrum (Oleaceae) en un relicto de selva subtropical en la Argentina. Rev. Chil. Hist. Nat. 66: 75-85. http://rchn.biologiachile.cl/pdfs/1993/1/Montaldo_1993.pdf
MONTTI, L., V. PIRIZ CARRILLO, J. GUTIÉRREZ-ANGONESE, N. I. GASPARRI, … & H. R. GRAU. 2017. The role of bioclimatic features, landscape configuration and historical land use in the invasion of an Asian tree in subtropical Argentina. Landsc. Ecol. 32: 2167-2185. https://doi.org/10.1007/s10980-017-0563-2.
NIINEMETS, Ü. 1999. Research review. Components of leaf dry mass per area - thickness and density - alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol. 144: 35-47.
https://doi.org/10.1046/j.1469-8137.1999.00466.x
PARKER, I. M., W. M. LONSDALE, K. GOODELL, M. WONHAM, … & L. GOLDWASSER. 1999. Impact: toward a framework for understanding the ecological effects of invaders. Biol. Invasions 1: 3-19.
https://doi.org/10.1023/A:1010034312781
PARKHURST, D. F. 1986. Internal leaf structure: a three-dimensional perspective. En: GIVNISH, T. J. (ed.), On the Economy of Plant Form and Function, pp. 215-250. Cambridge University Press, Cambridge.
PATTISON, R. R., G. GOLDSTEIN & A. ARES. 1998. Growth, biomass allocation and photosynthesis of invasive and native Hawaiian rainforest species. Oecologia 117: 449-459. https://doi.org/10.1007/s004420050680
PODAZZA, G. 2019. Especies vegetales exóticas: el establecimiento de plántulas de Ligustro sinense (Oleaceae) en selva basal fragmentada por explotación forestal (PN Aconquija, Tucumán) [online]. Disponible en: https://issuu.com/junazdg/docs/boletin_investigacion_y_monitoreo_noa_1 [Acceso: 20 Marzo 2023].
POORTER, H. & J. R. EVANS. 1998. Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 116: 26-37. https://doi.org/10.1007/s004420050560
PYŠEK, P., P. E. HULME, D. SIMBERLOFF, S. BACHER, … & D. M. RICHARDSON. 2020. Scientists’ warning on invasive alien species. Biol. Rev. 95: 1511-1534. https://doi.org/10.1111/brv.12627
RAWSON, H. M., J. E. BEGG & R. G. WOODWARD. 1977. The effect of atmospheric humidity on photosynthesis, transpiration and water use efficiency of leaves of several plant species. Planta 134: 5-10. https://doi.org/10.1007/BF00390086
REN, T., S. M. WERADUWAGE & T. D. SHARKEY. 2019. Prospects for enhancing leaf photosynthetic capacity by manipulating mesophyll cell morphology. J. Exp. Bot. 70: 1153-1165. https://doi.org/10.1093/jxb/ery448
REYNOLDS, C., N. VENTER, B. W. COWIE, D. MARLIN, … & M. J. BYRNE. 2020. Mapping the socio-ecological impacts of invasive plants in South Africa: Are poorer households with high ecosystem service use most at risk? Ecosyst. Serv. 42: 101075. https://doi.org/10.1016/j.ecoser.2020.101075
SCHULTE, M., C. OFFER & U. HANSEN. 2003. Induction of CO2-gas exchange and electron transport: comparison of dynamic and steady-state responses in Fagus sylvatica leaves. Trees 17: 153-163. https://doi.org/10.1007/s00468-002-0219-x
SIMBERLOFF, D. 2004. Community Ecology: Is it time to move on? Am. Nat. 163: 787-799. https://www.journals.uchicago.edu/doi/pdf/10.1086/420777
STRATTON, L. C. & G. GOLDSTEIN. 2001. Carbon uptake, growth and resource-use efficiency in one invasive and six native Hawaiian dry forest tree species. Tree Physiol. 21: 1327-1334. https://doi.org/10.1093/treephys/21.18.1327
TECCO, P. A., S. DÍAZ, D. E. GURVICH, N. PEREZ-HARGUINDEGUY, …& G. A. BERTONE. 2007. Facilitation and interference underlying the association between the woody invaders Pyracantha angustifolia and Ligustrum lucidum. Appl. Veg. Sci. 10: 211-218. https://doi.org/10.1111/j.1654-109X.2007.tb00519.x
TE BEEST, M., K. J. ESLER & D. M. RICHARDSON. 2015. Linking functional traits to impacts of invasive plant species: a case study. Plant Ecol. 216: 293-305. https://doi.org/10.1007/s11258-014-0437-5
TERASHIMA, I., Y. T. HANBA, Y. TAZOE, P. VYAS & S. YANO. 2006. Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J. Exp. Bot. 57: 343-354.
https://doi.org/10.1093/jxb/erj014
THEOHARIDES, K. A. & J. S. DUKES. 2007. Plant invasion across space and time: factors affecting nonindigenous species success during four stages of invasion. New Phytol. 176: 256-273. https://doi.org/10.1111/j.1469-8137.2007.02207.x
THÉROUX-RANCOURT, G., A. B. RODDY, J. M. EARLES, M. E. GILBERT, … & C. R. BRODERSEN. 2021. Maximum CO2 diffusion inside leaves is limited by the scaling of cell size and genome size. Proc. Royal Soc. B 288: 20203145. https://doi.org/10.1098/rspb.2020.3145
TOSCANO, S., A. FERRANTE, A. TRIBULATO & D. ROMANO. 2018. Leaf physiological and anatomical responses of Lantana and Ligustrum species under different water availability. Plant Physiol. Biochem. 127: 380-392. https://doi.org/10.1016/j.plaphy.2018.04.008
VAN KLEUNEN, M., E. WEBER & M. FISCHER. 2010. A meta‐analysis of trait differences between invasive and non‐invasive plant species. Ecol. Lett. 13: 235-245. https://doi.org/10.1111/j.1461-0248.2009.01418.x
VERGARA-TABARES, D. L., J. BADINI & S. I. PELUC. 2016. Fruiting phenology as a “triggering attribute” of invasion process: Do invasive species take advantage of seed dispersal service provided by native birds? Biol. Invasions 18: 677-687. https://doi.org/10.1007/s10530-015-1039-4
VILÀ, M., C. BASNOU, P. PYŠEK, M. JOSEFSSON, … & D. PARTNERS. 2010. How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front. Ecol. Environ. 8: 135-144.
https://doi.org/10.1890/080083
VOGELMANN, T. C. & H. L. GORTON. 2014. Leaf: light capture in the photosynthetic organ. En: HOHMANN-MARRIOTT, M. F (ed.), The Structural Basis of Biological Energy Generation, pp. 363-377. Springer, Netherlands.
WEBER, E. 2003. Invasive Plant Species of the World: A Reference Guide to Environmental Weeds. CAB International Publishing, Wallingford.
WHITEWOODS, C. D. 2021. Riddled with holes: Understanding air space formation in plant leaves. PLOS Biology 19: e3001475.
https://doi.org/10.1371/journal.pbio.3001475.g001
WRIGHT, I. J. & M. WESTOBY. 2002. Leaves at low versus high rainfall: coordination of structure, lifespan and physiology. New Phytol. 155: 403-416. https://doi.org/10.1046/j.1469-8137.2002.00479.x
WU, J., J. WANG, W. HUI, F. ZHAO, … & W. GONG. 2022. Physiology of plant responses to water stress and related genes: A Review. Forests 13: 324. https://doi.org/10.3390/f13020324
YANSEN, M. V. & F. BIGANZOLI. 2022. Las especies arbóreas exóticas en Argentina: caracterización e identificación de las especies actual y potencialmente problemáticas. Darwiniana 10: 80-97.
https://doi.org/10.14522/darwiniana.2022.101.1001
ZAMORA NASCA, L., L. MONTTI, R. GRAU & L. PAOLINI. 2014. Efectos de la invasión del ligustro, Ligustrum lucidum, en la dinámica hídrica de las Yungas del noroeste argentino. Bosque (Valdivia) 35: 195-205.
http://dx.doi.org/10.4067/S0717-92002014000200007
ZARLAVSKY, G. E. 2014. Histología Vegetal. Sociedad Argentina de Botánica, Buenos Aires.
ZHANG, S., D. FAN, Q. WU, H. YAN & X. XU. 2013. Eco-physiological adaptation of dominant tree species at two contrasting karst habitats in southwestern China. F1000Research 2: 122. https://doi.org/10.12688/f1000research.2-122.v2
ZHOU, J., Z. ZHANG, Y. ZHANG, Y. WEI & Z. JIANG. 2018. Effects of lead stress on the growth, physiology, and cellular structure of privet seedlings. PLoS ONE 13: e0191139. https://doi.org/10.1371/journal.pone.0191139
ZOU, J., W. HU, Y. LI, H. ZHU, … & Z. ZHOU. 2022. Leaf anatomical alterations reduce cotton’s mesophyll conductance under dynamic drought stress conditions. Plant J. 111: 391-405. https://doi.org/10.1111/tpj.15794
Publicado
Número
Sección
Licencia
Derechos de autor 2023 María Inés Mercado, Sebastian E. Buedo, Daniela A. González, Priscila A. Powell, Juan A. González

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
El Bol. Soc. Argent. Bot.:
- Provee ACCESO ABIERTO y gratuito inmediato a su contenido bajo el principio de que hacer disponible gratuitamente la investigación al público, lo cual fomenta un mayor intercambio de conocimiento global.

- Permite a los autores mantener sus derechos de autor sin restricciones.
- El material publicado en Bol. Soc. Argent. Bot. se distribuye bajo una licencia de Creative Commons Atribución-NoComercial-CompartirIgual 4.0 Internacional.







