10.31047/1668.298x.v40.n2.40923
Articulos
Relationships between soil chemical parameters and functional plant groups in fertility islands of the Arid Chaco (Argentina)
M. E. Torres
R. O. Coirini
A. M. Contreras
M. S Karlin
1 Torres, M. E. (ORCID: 0009-0006-5921-5120): Universidad Nacional de Córdoba, Facultad de Ciencias Exactas, Físicas y Naturales, Córdoba, Argentina. Maestría en Ciencias de la Ingeniería - Mención Ambiente. Rubén Coirini (ORCID: 0000-0003-4520-822X), Ana Contreras (ORCID: 0000-0002-3839-2586), Marcos Kariin (ORCID: 0000-0002-8642-4677): Universidad Nacional de Córdoba, Facultad de Ciencias Agropecuarias, Córdoba, Argentina. *Correspondence to: mkarlin@agro.unc.edu.ar
SUMMARY
The “fertility islands” are the result of soil partióles, water, nutrients, and biomass accumulation under their canopy. The study of soil and plant parameters in fertility islands is important for the redefinition of management strategies. The hypothesis is that Neltuma flexuosa and Larrea divaricata fertility islands in the Arid Chaco improve soil chemical properties under their canopies affecting the quality and quantity of forage. The objective is to evaluate soil chemical properties on the response of plants in fertility islands. The effect of nurse plants over soil chemical properties and their relation with functional plant groups was studied. Livestock carrying capacity (LCC) significantly increased under the canopy of N. flexuosa. Decreasers were positively related to the N. flexuosa canopy, and negatively to L. divaricata canopy. Soil organic carbon (OC) related positively with decreasers in N. flexuosa Islands, but negatively in L. divaricata islands where there seems to exist allelopathy effect. Extractable phosphorus correlated positively with decreasers.
Keywords: canopy, forage, Larrea divaricata, Neltuma flexuosa, nurse plants.
RESUMEN
Las “islas de fertilidad” son el resultado de la acumulación de partículas de suelo, agua, nutrientes y biomasa bajo su canopia. El estudio de parámetros de suelo y de vegetación en islas de fertilidad es importante para la redefinición de estrategias de manejo. La hipótesis es que las islas de fertilidad de Neltuma flexuosa y Larrea divaricata en el Chaco Árido mejoran las propiedades químicas del suelo bajo su canopia afectando la calidad y cantidad de forraje. El objetivo es evaluar las propiedades químicas del suelo sobre la respuesta de las plantas en islas de fertilidad. Se estudió la influencia de plantas nodrizas sobre propiedades químicas del suelo y su relación con grupos funcionales de plantas. La capacidad de carga ganadera (LCC) se incrementó significativamente bajo la canopia de N. flexuosa. Las especies decrecientes se relacionaron positivamente bajo canopia de N. flexuosa, y negativamente bajo L. divaricata. El carbono orgánico del suelo (OC) se relacionó positivamente con las especies decrecientes en islas de N. flexuosa, pero negativamente en las islas de L. divaricata donde aparentemente ocurre alelopatía. El fósforo extractable se correlacionó positivamente con las especies decrecientes.
Palabras clave: canopia, forraje, Larrea divaricata, Neltuma flexuosa, plantas nodrizas.
INTRODUCTION
The United Nations Convention to Combat Desertification (UNCCD, 2015) defined land degradation neutrality as “a state whereby the amount and quality of land resources necessary to support ecosystem functions and services and enhance food security remain stable or increase within specified temporal and spatial scales and ecosystems”.
In contrast, an ecosystem is subjected to livestock and forestry overuse when plant biomass is consumed by livestock or it is extracted by human beings during a determinate amount of time, surpassing forage or forestry biomass productivity for that period (Wood and Carvalho, 2000). An ecosystem overloaded with livestock negatively affects the normal growth of plants, seedlings, and sprouts of the most palatable species (Teich et al., 2005), reducing their abundance and indirectly increasing the abundance of less palatable species. Livestock and forestry exploitation may also affect soil, directly through soil compaction, reducing soil hydraulic conductivity, or indirectly by altering the vegetation responsible for cycling nutrients and soil mulching (Kremers and Boosten, 2018; Karlin et al., 2019).
In arid and semiarid lands, plants and especially trees, are considered the best regulators in terrestrial natural ecosystems (Karlin, Galán et al., 2013). The reduction of plant cover increases bare soil, affecting the physical, chemical and biological attributes by alteration of erosion controls and mineralization (Naldini et al., 2021).
Ecosystems in arid and semiarid lands show considerable spatial heterogeneity regarding soil, plants, and fauna due to both natural and anthropic causes. Anthropic causes promote ecosystems with a shrubby matrix and bare soil of reduced physicochemical fertility. Trees and shrubs are sparse as a consequence of a sustained extraction within the ecosystems. They usually enhance environmental conditions under canopy compared to intercanopy (Tongway and Ludwig, 2005; Karlin, Coirini et al., 2021; Karlin, Zapata et al., 2021). Such areas are known as “fertility islands” and are the result of an important accumulation of soil particles, water, nutrients and biomass under the canopy (Rossi and Villagra, 2003; Ridolfi et al., 2008).
It has been proved that trees understorey modify the physical, chemical, and biological dynamics in ecosystems, regulating the amount and availability of nutrients, water and the functioning of living organisms, creating special micro-environments compared to those from the intercanopy (Dohn et al., 2013). Nevertheless, studies about the ¡nfluence of shrubs over fertility islands are sometimes contradictory; environmental effects seem to depend on the species, shrub size, and environmental context (Thompson et al., 2005; Qu et al., 2018; Ward et al., 2018).
The ecosystems in the Argentinian Arid Chaco are not the exception to the aforementioned degradation processes. In the last century, these have suffered a critica! reduction of productivity and management regulation, which has led to the transformation of primary forests into scrubs. The latter are a product of degradation, selective extraction and livestock facilitation, usually defining fertility islands with isolated nurse species such as mesquite (Neltuma flexuosa (DC.) C. E. Hughes & G. P Lewis) or creosotebush (Larrea divaricata Cav.) (Karlin, Karlin et al., 2013). The abundance of forage species depends directly on livestock grazing frequency and intensity, and indirectly on soil and micro-environment conditions. These species can be categorized as “decreasers”, “increasers” or “invaders” according to Dyksterhuis' classification (Díaz, 2007). The abundance of such species defines the productivity in grazing systems.
Currently, due to changes in productive and conservation paradigms, the role of trees and shrubs in the regulation of environmental and socioeconomic conditions is being revisited. The study of the changes in biotic and abiotic variables inside and outside fertility islands is of vital importance in arid and semiarid ecosystems for the redefinition of management, rehabilitation, and conservation strategies (Varela et al., 2017).
The hypothesis is that N. flexuosa and L. divaricata fertility islands in the Arid Chaco improve soil chemical properties (organic carbon, phosphorus, electrical conductivity, and pH) under their canopies compared to the intercanopy, positively affecting the quality and quantity of forage.
The objective is to evaluate soil chemical properties (organic carbon, extractable phosphorus, electrical conductivity, and pH) on the biomass response and plant frequencies within and outside the canopy of N. flexuosa and L. divaricata in fertility islands.
MATERIALS AND METHODS
Study sites
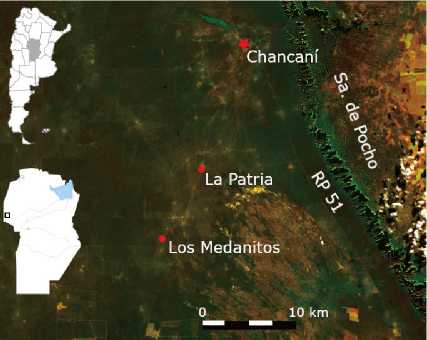
Two plots corresponding to livestock closures on recovery were selected in the locations of La Patria (31° 32' 00” S, 65° 30' 20” W; 288 m a.s.l.) and Los Medanitos (31° 36' 00” S, 65° 33' 00” W; 287 m a.s.l.), both located in the geomorphological unit of dune fields and areas of sand blankets or isolated dunes (Carignano et al., 2014). They are located 13 and 23 km southwest of the Chancaní location, respectively, in the Pocho Department at the westernmost part of the Province of Córdoba, Argentina (Figure 1).

Figure 1: Relative location of the plots under study. Sentinel 2 L2A image, 1/7/2022, false color (R: B12, G: B6, B: B2).
According to Koppen's climatic classification (Beck et al., 2018), the study area has a warm semiarid climate (BSh). Precipitations range between 350 and 650 mm yearly (Karlin, 2012).
The plot located in La Patria (1.9 ha) is a scrub of L. divaricata with a history of intense grazing before its closure in 2015. The plot in Los Medanitos (2.1 ha), also closed in 2015, is a woodland of N. flexuosa with a history of moderate grazing and wood extraction. Both plots were closed until September 2020 and grazed in low intensities after plant fructificaron (April-May) between 2017 and 2020.
Experimental design
Soil chemical properties and their relation with functional plant groups were studied within and outside the canopy of N. flexuosa and L. divaricata nurse plants. In each plot, five individuals of each nurse plant were selected, on the condition that they were isolated forming fertility islands (Figure 2). Transects were extended from the island's centre towards the intercanopy, all oriented to the north. Transects had a length of two times the canopy radius (r) of each nurse plant. Each individual had a different canopy radius.
Soil sampling
A superficial layer 10 cm deep was extracted for simple soil samples under the canopy (UC), at the canopy limit (LC) and in the intercanopy (IC) in each fertility island (n = 60).
Soil samples were pulverized, sieved and air-dried at 60 °C in a forced-air chamber for three days. Soil organic carbon content (OC) was determined by the Walkley and Black method (Nelson and Sommers, 1996); extractable phosphorus (EP) by the Bray and Kurtz N°1 method (1945); electrical conductivity (EC) by conductometry in the supernatant soil/water=1:1, and pH by potentiometry. Bray and Kurtz N°1 is a common method to determine soil pH near neutrality, below 7.5 (Sims, 2000; Mustapha et al., 2022). It works well for carbonate-lacking soils. However, Ebeling et al. (2008) recommend it for pH 7 to 8, arguing that such ranges do not affect phosphorus extraction. The 0-10 cm deep soil samples in this study lack significant carbonate content since they did not react to hydrochloric acid. Both EC and pH were measured with a Hanna HI98129 pHmeter-conductimeter.
Plant survey
The modified Point Quadrat method was applied (Passera et al., 1986), measuring plant cover during early winter (June, 2019), late spring (December, 2019) and late summer (March, 2020). Two sectors were identified along the transects; the first (UC) from the central point of the fertility island to the limit of the canopy, and the second (IC) from the limit of the canopy to a point located at a length of two times the canopy radius (2r).
The plants found along the transects were identified, obtaining their frequencies by calculating the specific contribution by contact (SCC) using a 1.5 m long by 0.003 m wide needle. SCC (1) is the quotient between the number of contacts of each species (C) and the sum of contacts of all surveyed species along a transect (bare soil was not accounted). The points along each transect of variable length were taken every 0.20 m.
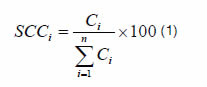

Figure 2: Fertility islands of a) N. flexuosa and b) L. divaricata. UC: under canopy; LC: canopy limit; IC: intercanopy
The surveyed species were grouped into three categories according to Dyksterhuis (1949): decreasers, increasers and invaders. Decreasers reduce their frequencies with grazing intensity; increasers increase their frequencies with a reduction in the decreaser's competition, but they may decrease if grazing continues after the disappearance of the decreasers; invaders are all the ungrazed or unbrowsed species, usually represented by annuals and woody species other than nurse species. The SCC for each species was summed according to the category, obtaining the plant frequencies per category.
Particularly, the frequencies of the lichen Selaginella sellowii were discriminated, since it is an important plant indicator for degradation intensity.
Livestock carrying capacity determination
The Specific Quality Indexes (SQI) were considered for each one of the identified species with the Point Quadrat method. The indexes were obtained from Passera and Borsetto (1986) and Karlin (2013). The Pastoral Value (PV) was calculated for each transect and treatment through the following formula (2):
PV = 0.1 x Σ (SCC, x SQI) x FC (2)
FC is the forage cover calculated through the sum of frequencies of each forage species Y found at each point along the transects. Considering that 100 unities of pastoral value (UPV) can maintain an animal unit (AU) per hectare, PV was transformed into livestock carrying capacity units (LCC) measured as AU ha-1 (3).
LCC = PV/100 (3)
An AU is equivalent to the annual average nutritional requirements of a 400 kg cow gestating and breeding a calf until weaning at 6 months old and 160 kg, including the forage consumed by the calf (Passera et al., 1986).
Statistical analysis
Shapiro-Wilks normality tests were performed. Those parameters that were not significant at a p-value=0.0001 or showed W* values larger than 0.90 were analyzed through ANOVA tests, while those with p<0.0001 or W*<0.90 were analyzed through Kruskal-Wallis tests.
Tests were discriminated by nurse species (N. flexuosa y L. divaricata), by plot and, in the case of plant frequencies, by season (winter, spring and summer). For plant frequencies, only UC and IC treatments were considered.
Pearson's correlation coefficients between soil and plant parameters were obtained. Only the biological parameters from March were analyzed since they reached their highest expression by the end of the summer. LC treatment was not considered since it had no associated plant data.
All the statistical analyses were performed with the InfoStat statistical software (Di Rienzo et al., 2020).
RESULTS AND DISCUSSION
Normality tests
According to Shapiro-Wilks test results, OC, pH and LCC can be considered with normal distribution. The rest, EP, EC, and the frequencies of the functional plant groups did not show normal distributions; therefore, they were analyzed by Kruskal-Wallis tests.
Soil parameters
The results of the soil chemical parameters (OC, EP, EC and pH) are shown in Figure 3.
Soil organic carbón
In Figure 3a, data show that in UC, in each fertility island category (N. flexuosa y L. divaricata), OC is significantly higher than in LC and IC. Soil organic carbon declines from the centre of the island towards the intercanopy.
The average contents of soil organic carbon in
N. flexuosa islands were 1.22 % for UC, 0.86 % for LC and 0.70 % for IC; while in L. divaricata islands its values were 1.10 % for UC, 0.79 % for LC and O. 69 % for IC.
No significant differences were found between islands when treatments were compared separately (UC: p - value= 0.2689; LC: p - value = 0.4496; IC: p - value= 0.9059), despite that it is expected that the litter contribution from mesquite would be higher than that of creosotebush. Similar results were found in woodlands from the Monte region (Argentina) by comparing the ¡nfluence of the canopy between trees and shrubs (Karlin, Zapata et al., 2021).
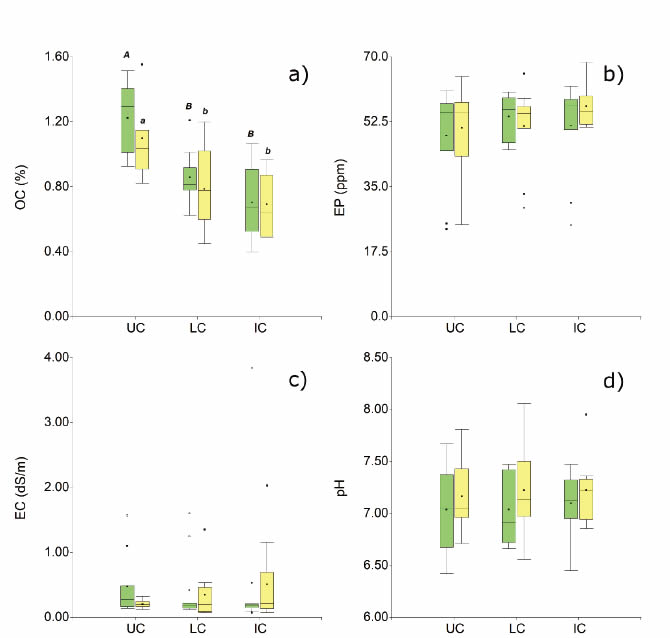
Figure 3: Box-plots of the soil Chemical parameters: a) soil organic carbón (OC); b) extractable phosphorus (EP); c) electrical conductivity (EC); d) pH. UC: under canopy; LC: canopy limit; IC: intercanopy. Green bars: N. flexuosa islands; yellow bars: L. divaricata islands. Different letters indicate statistically significant differences (ANOVA LSD Fisher test, a=0.05)
Soil organic carbon is concentrated within the fertility islands, basically due to litter falling from the trees or shrubs, especially in N. flexuosa islands. On the other hand, trees and shrubs would perform as sediments and litter traps transported by water or wind, enriching the understorey soil even more with organic carbon (Aber and Melillo, 2001).
Regarding the use history of the plot, significant differences were only found in the IC treatment between La Patria (history of intense grazing) and Los Medanitos (history of moderate grazing) (OC: 0.59 % vs. 0.81 %, respectively; ANOVA LSD Fisher, p= 0.0090), even though both plots were excluded from livestock for three years before the survey. Regarding the other treatments, LC presented only marginal differences (0.74 % vs. 0.90 %, respectively; ANOVA LSD Fisher, p= 0.0846) while UC did not show significant differences (1.13 % vs. 1.19 %, respectively; ANOVA LSD Fisher, p=0.6062). These results can be contrasted with those obtained by Abril et al. (2005), who compared situations of intense grazing with ungrazed sites in the same region (Arid Chaco). They concluded that carbón content in plots with 52 % of bare soil were significantly poorer (38 % less soil organic carbon) than the control corresponding to a climaxic Aspidosperma quebracho-blanco Schltdl. forest. However, the effects of fertility islands were not studied by these authors.
From these results it is evidenced that degradation effects may be buffered by trees and shrubs, differing from the areas lacking their protection and organic matter enrichment.
Extractable phosphorus
No significant differences (a= 0.05) were observed between treatments for extractable phosphorus, either in N. flexuosa islands or in L. divaricata (Figure 3b). The p-values obtained were p= 0.6769 and p= 0.6523, respectively.
No significant differences were found between plots, despite differences in soil organic contents, and considering that phosphorus can also be added through organic matter (UC, p= 0.5452; LC, p= 0.2897; IC, p= 0.2265).
The lack of statistical differences between treatments shows that phosphorus is controlled by pedogenetic characteristics independently from the ¡nfluence of nurse plants, i.e. the organic source is practically irrelevant. These soils, derived from parental materials enriched in phosphorus, offer high concentrations of this nutrient, compared to other areas or regions (Hang et al., 2015). Thus, while in this study no statistical differences were found, other authors (Gao et al., 2022) discovered evident differences in extractable phosphorus content in fertility islands from hyperarid deserts (Taklimakan desert, China; average precipitations of 43 mm year-1) favouring sites under canopy. However, they also proved that some inorganic fractions showed no concentration differences between treatments in the studied fertility islands (Alhagi sparsifolia Shap., Karelinia caspia (Pall.) Less., Tamarixramosissima Ledeb.).
Similarly to the present study, Roos and Allsopp (1997) found that when nurse trees (in their study, Vachellia sieberiana (DC.) Kyal. & Boatwr. = Acacia sieberiana) are dispersed in low densities within a savanna matrix, these do not offer extra phosphorus under the canopy and therefore the organic fractions are negligible compared to inorganic fractions. Ding and Eldrige (2021) suggest that high concentrations of inorganic phosphorus, low nurse plant cover, and sparse fertility islands promote poorer microbial communities which produce the phosphatase enzyme responsible for organic phosphorus mineralization.
Ochoa Hueso et al. (2011) point out that soil phosphorus concentrations respond to changes in the plant cover, the kind of plant species, the dominant plant species, and the predominant functional plant group.
Possibly net changes in organic phosphorus may be reasonably small related to the total reservoir pool. Consequently, some more observations over several years are needed to detect significant effects under rehabilitation conditions in degraded environments.
Electrical conductivity
No significant differences (a= 0.05) were found between treatments for electrical conductivity in any of the fertility island categories (p= 0.2386 and 0.9169 for N. flexuosa and L. divaricata, respectively) (Figure 3c). These results differ from those obtained by Rossi and Villagra (2003), who found that electrical conductivity was higher under the canopy of N. flexuosa islands in the Natural Reserve of Ñacuñán (phytogeographical province of Monte, Mendoza, Argentina), with drier conditions than the Arid Chaco.
By comparing the UC treatment between N. flexuosa and L. divaricata, differences (p= 0.0959) were found, with 0.47 dS m-1 against 0.20 dS m-1, respectively. This situation might be caused by the pedestal effect in L. divaricata, offering higher infiltration rates and reduced bulk densities compared to those of N. flexuosa (basic infiltration: N. flexuosa= 38.37 mm h-1, L. divaricata= 75.57 mm h-1; soil bulk densities: N. flexuosa= 1.26 Mg m-3, L. divaricata= 1.20 Mg m-3; according to Karlin, Coirini et al., 2021), favouring the salt leaching under the canopy.
Significant differences were also found in the IC between plots, with higher electrical conductivities in La Patria, more degraded than Los Medanitos (0.90 vs. 0.15 dS m-1; Kruskal-Wallis, p= 0.0080). In contrast, only marginally significant differences were found in LC (p= 0.0959), while no significant differences were found in UC (p= 0.1208). Zarekia et al. (2012) found that grazing intensity in Iran grasslands affects soil electrical conductivity. Under continuous and intense grazing, the soil is exposed to solar irradiation, promoting evaporation and the ascent of soluble salts, compared with sites under rotative and light grazing, aiding herbaceous plant cover.
pH
No significant differences were found between treatments for this parameter (p= 0.9024 and 0.9126 for N. flexuosa and L. divaricata, respectively; Figure 3d). These results evidence that canopy has no effect over this parameter. Significant differences were neither found for UC between nurse plants (p-value= 0.4486). Perhaps pH is not a reliable indicator for this kind of system.
When comparing plots, these showed significant differences for all three treatments (UC, p= 0.0002; LC, p= 0.0024; IC, p= 0.0008), evidencing more acidic pH values in Los Medanitos (moderate grazing history) compared with La Patria (intense grazing history) (6.84 vs. 7.36; 6.89 vs. 7.37; 6.95 vs. 7.37, respectively). With the lack of canopy effect by the nurse plants, differences may be due to two causes: the ¡nfluence of the intensity use and herbaceous cover, or differences in the soil parental material. Both Zarekia et al. (2012) and Bakhshi et al. (2020) found that in Iranian arid and semiarid grasslands, pH showed no significant differences according to different grazing intensities. Likewise, in the Argentinian Semiarid Chaco district, Abril and Bucher (1999) did not find differences in this parameter by comparing different degradation situations due to overgrazing. Probably, soil pH differences between plots could be caused by differences in parental materials, although other causes should not be discarded. The parental material in Los Medanitos is formed by fluvial sands and silts with interspersed silty sediments, while silty loessic sediments, with calcium carbonate, can be found in La Patria (Martino et al., 2020). Therefore, despite not reacting with hydrochloric acid in the topsoil of either soil sample, carbonates may have some influence on soil pH differences.
Biological parameters
Livestock carrying capacity
Livestock carrying capacity data were analysed for all three seasons, comparing treatments (UC and IC) and fertility islands (N. flexuosa and L. divaricatá). There were only significant differences (p=0.0858) between UC and IC in N. flexuosa islands during March, when biomass is fully developed (Figure 4).
By comparing among seasons, LCC during March is higher and more statistically significant than the rest of the seasons (p<0.0001) (Figure 4). The canopy of nurse plants ¡nfluences the development of grasses after the rainfall season, with higher temperaturas and radiation, and in moments in which soil is recharged with water, producing maximum biomass accumulation. However, outside canopy influence grasses are also more developed during March compared to June and December.
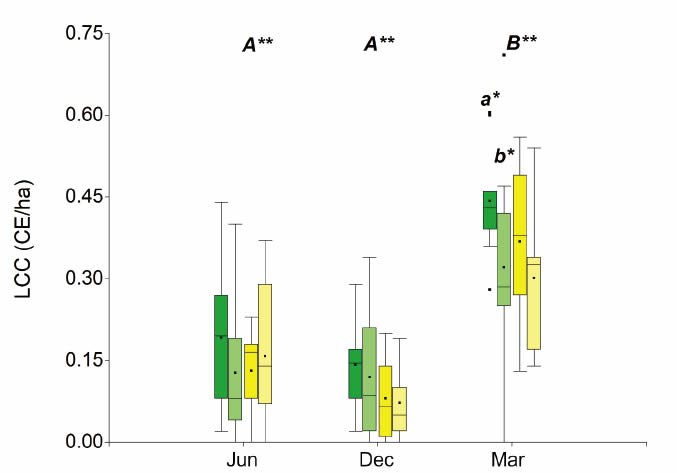
Figure 4: Box-plots of the livestock carrying capacity (LCC). Green bars: N. flexuosa islands; yellow bars: L. divaricata islands. Dark colours: UC; light colours: IC. Different letters indicate statistically significant differences. Lowercase: between treatments; uppercase: between seasons (ANOVA LSD Fisher test, **: a=0.05; *: a=0.1)
Functional plant groups: decreasers, increasers and invaders
The distribution of species according to functional groups (decreasers, increasers and invaders) is shown in Figure 5.
The species classified as decreasers according to field dynamics were Setaria spp., Leptochloa crinita (Lag.) PM. Peterson & N.W. Snow, Digitaria spp., Pappophorum caespitosum R.E. Fr. These are characterized to reduce their frequencies under growing grazing intensities by ruminants like sheep or goats.
Increasers, defined by the species Chloris castilloana Lillo & Parodi, Neobouteloua lophostachya (Griseb.) Gould, Aristida mendocina Phil., Sporobolus pyramidatus (Lam.) Hitchc., increase their frequencies according to the reduction of decreasers, but at higher grazing intensities, they also reduce their frequencies.
Invaders are represented by annual species such as Aristida adscensionis L., straws such as Jarava spp. and Nassella spp., broadleaf species such as Pseudabutilon virginianum (Cav.) Fryxell, Alternanthera pungens Kuntz and Talinum spp., and shrubby species such as Lycium tenuispinosum Miers, Senna aphylla (Cav.) H.S. Irwin & Barneby, Atamisquea emarginata Miers ex Hook. & Arn., Senna acanthoclada (Griseb.) H.S. Irwin & Barneby, and Condalia microphylla Cav. These increase their frequencies according to the reduction of decreasers and increasers. Eventually, these species may disappear under extreme degradation conditions, evidencing pedestals covered with Selaginella sellowii Hieron. within a matrix of bare soil (Karlin, Karlin et al., 2013; Figure 2b). Invaders are not preferred by the animals due to their high toxicity, low palatability or prickliness. Such species appear in situations under high grazing pressure or erosion signs, indicating ecological disturbances.
According to Kruskal-Wallis tests, the only functional groups that offer significative differences between treatments (UC and IC) at a= 0.05 are the decreasers in N. flexuosa islands in each season (June: 0.0394; December: 0.0364; March: 0,0492).
Under the canopy of N. flexuosa, the recovery processes seemed to be accelerated. Decreasers have been favoured by the nurse plants (Figure 5a), and possibly the seed bank suffered fewer losses of decreasers compared to spaces in the intercanopy. In the intercanopy, the frequency of decreasers is lower maybe due to adverse biophysical conditions, such as reduced soil moisture content, higher air temperature, higher radiation (Karlin, Coirini et al., 2021) or reduced nutrient contents.
Under reduced grazing pressure, decreasers might reduce the frequency of straws (Jarava spp. and Nassella spp., representing invaders) through interspecific competence (Crawley, 1990).
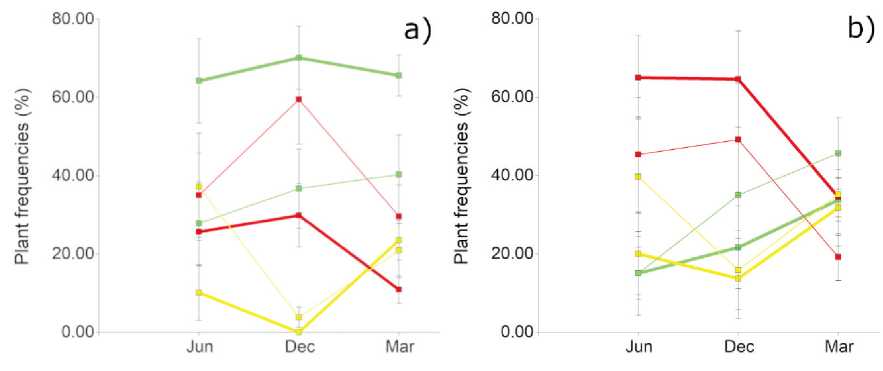
Figure 5: Frequency (%) of the plant functional groups in N. flexuosa (a) and L. divaricata (b) fertility islands. Green lines: decreasers; yellow lines: increasers; red lines: invaders. Thick lines: UC; thin lines: IC. Bars indicate the standard error
L. divaricata nurse plants did not show suitable conditions for decreasers under their canopy compared to the intercanopy (Figure 5b). On the contrary, IC has a frequency of decreasers 35.8 % higher than UC, although no significant differences were found (p=0.5810). Allelopathic effects of the creosotebush may affect the growth of the species under their influence (Mahall and Callaway, 1991). However, the lack of significance requires more detailed studies. Similarly, studying L. tridentata as nurse species, Schafer et al. (2012) found that annual species increased their abundance along a gradient from sites under canopy towards the interspaces, without their influence. Soil water during the rainy season could dilute or leach allelopathic substances, improving the frequency of decreasers (Lei, 2010), as seen in Figure 5b.
According to the results, N. flexuosa might act as a facilitator, while L. divaricata could be an inhibitor (Dohn et al., 2013). Nevertheless, it is evident in both cases that precipitations and temperature favour the growth and frequency of decreasers and increasers, compared to invaders. While frequencies of decreasers + increasers rise, frequencies of invaders fall, and vice versa. Such relation is evidenced in the following linear regression (4):
InvF = 96.69 - 0.96 x (DecF + IncF) (R2 = 0.93; CpMallows = 1620.30; p-value < 0.0001) (4)
Selaginella sellowii is a “cicatrizing” species from arid lands accompanied by other biological organisms such as cyanobacteria, algae, lichens, mosses and microfungi, forming biocrusts (Figure 2b). Its cover changes depending on the nurse species, season or treatment, as shown in Figure 6.
Although no significant differences were found between islands, partitioned by season and treatment (a= 0.05), frequencies of Selaginella under the influence of L. divaricata canopy during June and December are far more important than under N. flexuosa. Nevertheless, standard deviations are quite important during both seasons.
On the other hand, frequencies of Selaginella crusts drastically drop during March, when the cover of other species rises markedly and the vegetative structures of this pteridophyte cease to be visible. This species has an important role in soil regulation. It creates a protective living crust in highly degraded sites. It can retain up to three times its weight (Potsch and Arens, 1949) and reduce soil evaporation, controlling erosion and nutriment losses (Castillo-Monroy et al., 2016), stabilizing pedestals formed by overgrazing and sedimentation.
Soil-plant relationships
Pearson's correlation coefficients are shown in Table 1, discriminated by fertility island.
It is difficult to predict the behaviour of the plant under the canopy in either nurse plant by using soil chemical variables independently as proxies. However, some significant relations between parameters can be identified. For instance, it is expected for soil organic carbon to be closely correlated with mineralized nitrogen available for plant growth (Carranza et al., 2012). Nevertheless, soil organic carbón does not correlate significantly with LCC (as a proxy for plant biomass) during March in either of the fertility islands (Table 1).
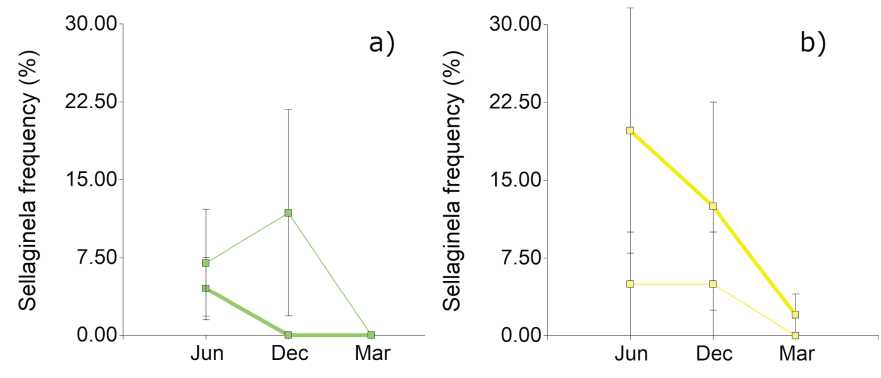
Figure 6: Frequencies of Sellaginella sellowii; in a) N. flexuosa fertility islands (green lines) and b) L. divaricata fertility islands (yellow lines); UC (thick lines) and IC (thin lines). Bars indicate the standard error
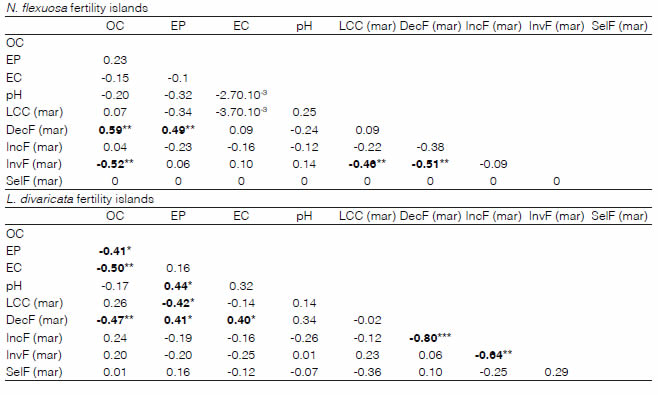
Table 1: Pearson’s correlation coefficients between soil and plant parameters (the latter, only for late summer season) for each category of fertility islands. OC: soil organic carbon; EP: soil extractable phosphorus; EC: electrical conductivity; LCC: livestock carrying capacity; DecF: frequencies of decreasers; IncF: frequencies of increasers; InvF: frequencies of invaders; SelF: frequencies of Selaginella; (mar): March. In bold, significant correlations: ***p < 0.001; **0.001 < p < 0.05; *0.05 < p < 0.1
As seen before, soil organic carbon is higher in the UC treatment in both island categories (Figure 3a). Nevertheless, it is positively related to decreasers in N. flexuosa islands, but negatively in L. divaricata islands (Table 1). It means that within L. divaricata islands, decreasers are inhibited by the ¡nfluence of this nurse species, instead of being promoted by the higher carbon contents under their canopy, compared to the intercanopy. This reinforces the idea that creosotebush may produce allelopathic effects (Mahall and Callaway, 1991). This effect does not occur under the influence of N. flexuosa.
The Bray and Kurtz N°1 method to estimate extractable phosphorus (Sims, 2000) is a measure of the potential availability of phosphates in non-clayey, non-calcareous, neutral or slightly acidic soils by the plants (pH < 7.5, as occurs in the study sites, Figure 3d). For this study, extractable phosphorus had positive and significant correlations with the frequencies of decreasers; however, it had negative and significant correlations with LCC (p=0.07 in L. divaricata islands; p=0.01 when both islands are combined). Therefore, extractable phosphorus is inversely related to grass biomass (proxied by LCC, significantly higher under the canopy of N. flexuosa; Figure 4) as also found by Chidumayo (1997) and Riginos et al. (2009) for dry savannas. Riginos et al. (2009) suggest that maybe phosphorus is more intensely extracted by nurse trees or shrubs, under their canopies; therefore, this may reduce available phosphorus. This effect might be possible for L. divaricata because it has superficial roots and may compete with herbaceous plants, but improbable for N. flexuosa, with deeper and extended rooting (Villagra et al., 2011). The other possible explanation is that phosphorus content could be affected by the understory plants. However, no significance was found for EP between treatments in favour of the UC (Figure 3b), and possibly the correlation between EP and LCC is independent of nurse plant influence. Besides, phosphorus content show relatively high concentrations (always superior to 23 ppm and averaging 52 ppm), above the critical threshold for the normal development of plants. This threshold for plants ranges between 15 and 25 ppm, depending on soil characteristics, plants and environmental conditions (Beegle, 2005).
Regarding electrical conductivity, this parameter only correlates positively and marginally with decreasers in L. divaricata islands, since both parameters increase their valúes with the distance from the centre of the island. This effect is probably unimportant since EC do not show significant differences between treatments, and such values should not affect plant performance (Figure 3c). pH correlates with neither plant parameter.
Functional plant groups have predominantly negative relations among them. Decreasers compete with both increasers (highly significant in L. divaricata islands) and invaders (moderately significant in N. flexuosa islands). Increasers and invaders compete significantly only in L. divaricata islands (Table 1).
It is frequently addressed that decreaser species usually produce more forageable biomass than other categories, especially compared with increasers (Díaz, 2007). However, in this study higher decreaser frequencies do not necessarily mean an increase in the carrying capacity. LCC correlates negatively with invaders in N. flexuosa islands. As seen in Figure 5, invaders have higher frequencies under the L. divaricata canopy compared with N. flexuosa. Invaders seem to be facilitated by creosotebush and inhibited by mesquite, possibly due to light intensity, which is reduced in the latter, according to Karlin, Coirini et al. (2021). Nevertheless, these authors found that the correlation between invaders and light intensity under canopy was not significant.
The frequencies of Selaginella are null under the canopy of N. flexuosa, while it develops better under the L. divaricata canopy. This may be due to differences in plant and litter cover since it is considered that the development of this pteridophyte is reduced under closed canopy (by the nurse plant and/or understory plants) or due to high litter concentration conditions (Castillo-Monroy et al., 2016). Both plant cover and litter content were higher under the canopy of N. flexuosa compared with L. divaricata (Karlin, Coirini et al., 2021).
CONCLUSIONS
N. flexuosa canopy has significant effects on livestock carrying capacity during March, when plants show their maximum expression. On the contrary, the canopy of L. divaricata has no significant effect on this variable. The frequency of decreasers improves under N. flexuosa canopy, compared to L. divaricata canopy. There is no clear relationship between functional plant groups and biomass productivity.
Soil chemical variables relate differently to biological variables. Even though both OC and LCC parameters were higher under the canopy of both fertility islands, these parameters did not relate significantly. LCC and EP correlated negatively, but only significantly in L. divaricata islands, perhaps due to the fact that phosphorus tended to increase towards IC and LCC tended to decrease. LCC showed no significant relationships with either EC or pH.
Soil chemical variables relate better with the frequencies of functional plant groups; however, relations depended on the island. OC related positively with decreasers in N. flexuosa islands, but negatively in L. divaricata islands. In the latter, there seemed to exist some sort of allelopathy effect that inhibited potential positive effects of OC over this functional plant group. EP correlated positively with decreasers and there seemed to be some sort of control over this kind of functional plant group.
The behaviour of the other functional plant groups seemed to depend on competing effects among them, except for Selaginella which seemed to depend on other factors different than those evidenced in this paper, such as plant cover or litter cover. Further studies should be done in the future to get some more detailed relations.
The characteristics of the islands promote different effects on the vegetation under their ¡nfluence. LCC and the frequency of decreasers are enhanced by the canopy of N. flexuosa offering higher digestible biomass for livestock. Such nurse species cover should be promoted to improve productivity and environmental quality, altogether with management practices that tend to recover grasslands through closures and rotative grazing.
ACKNOWLEDGEMENTS
We thank J. Heredia and N. Oviedo for offering part of their farms as study plots. This project was supported by Secyt, Universidad Nacional de Córdoba, Argentina, Project No. 33620180101410CB.
Fecha de recepción: 12/04/2023
fecha de aceptación: 07/12/2023
REFERENCES
Aber, J. D. and Melillo, J. M. (2001). Terrestrial ecosystems. Brooks Cole.
Abril, A., Barttfeld, P. and Bucher, E. H. (2005). The effect of fire and overgrazing disturbes on soil carbon balance in the Dry Chaco forest. Forest Ecology and Management, 206(1-3), 399-405. https://doi. ora/10.1016/i.foreco.2004.11.014
Abril, A. and Bucher, E. H. (1999). The effects of overgrazing on soil microbial community and fertility in the Chaco dry savannas of Argentina. Applied Soil Ecology, 12(2), 159-167. https://doi.org/10.1016/ S0929-1393(98)00162-0
Bakhshi, J., Javadi, S. A., Tavili, A. and Arzani, H. (2020). Study on the effects of different levels of grazing and exclosure on vegetation and soil properties in semi-arid rangelands of Iran. Acta Ecológica Sinica, 40(6), 425431. https://doi.org/10.1016/j.chnaes.2019.07.003
Beck, H. E., Zimmermann, N. E., McVicar, T R., Vergopolan, N., Berg, A. and Wood, E. F. (2018). Present and future Koppen-Geiger climate classification maps at 1-km resolution. Scientific Data, 5, 180214. https://doi. org/10.1038/sdata.2018.214
Beegle, D. (2005). Assessing soil phosphorus for crop production by soil testing. In: Sims, T and Sharpley, A. N. Phosphorus: agriculture and the environment. Agronomy Monograph No. 46 (pp. 123-143). American Society of Agronomy, Crop Science Society of America, Soil Science Society of America. https:// doi.org/10.2134/agronmonogr46.c5
Bray, R. H. and Kurtz L. (1945). Determination of total, organic and available forms of phosphorus in soils. Soil Science, 59, 39-45.
Carignano, C. A., Krohling, D., Degiovanni, S. and Cioccale, M. (2014). Geomorfología. In: Relatorio del XIX Congreso Geológico Argentino. Geología de Superficie (pp.747-821).
Carranza, C., Noe, L., Merlo, C., Ledesma, M. and Abril, A. (2012). Efecto del tipo de desmonte sobre la descomposición de pastos nativos e introducidos en el Chaco Árido de la Argentina. RIA. Revista de Investigaciones Agropecuarias, 38(1), 97-107. https:// www.redalvc.org/pdf/864/86423614016.pdf
Castillo-Monroy, A. P., Benítez, Á., Reyes-Bueno, F., Donoso, D. A. and Cueva, A. (2016). Biocrust structure responds to soil variables along a tropical scrubland elevation gradient. Journal of Arid Environments, 124, 31-38. https://doi.org/10.1016/j.jaridenv. 2015.06.015
Chidumayo, E. N. (1997). Annual and spatial variation in herbaceous biomass production in a Zambian dry miombo woodland. South African Journal of Botany, 63(2), 74-81. https://doi.org/10.1016/S0254-6299(15)30706-7
Crawley, M. J. (1990). Rabbit grazing, plant competition and seedling recruitment in acid grassland. Journal of Applied Ecology, 27, 803-820. https://doi.org/10.2307/2404378
Di Rienzo, J., Casanoves, F., Balzarini, M., Gonzalez, L., Tablada, M. and Robledo, C. (2020). InfoStat versión (2020). Grupo InfoStat, FCA, Universidad Nacional de Córdoba.Díaz, R. O. (2007). Utilización de pastizales naturales.Editorial Brujas.
Ding, J. and Eldridge, D. J. (2021). The fertile island effect varies with aridity and plant patch type across an extensive continental gradient. Plant and Soil, 459, 173-183. https://doi.org/10.1007/s11104-020-04731-w
Dohn, J., Dembélé, F., Karembé, M., Moustakas, A., Amévor, K. A. and Hanan, N. P. (2013). Tree effects on grass growth in savannas: competition, facilitation and the stress-gradient hypothesis. Journal of Ecology, 101(1), 202-209. https://doi.org/10.1111/1365-2745.12010
Dyksterhuis, E. J. (1949). Condition and management of range land based on quantitative ecology. Journal of Range Management Archives, 2(3), 104-115. https://journals.uair.arizona.edu/index.php/jrm/article/viewFile/4330/3941
Ebeling, A. M., Bundy, L. G., Kittell, A. W. and Ebeling, D. D. (2008). Evaluating the Bray P1 test on alkaline, calcareous soils. Soil Science Society of America Journal, 72(4), 985-991. https://doi.org/10.2136/ sssaj2006.0347
Gao, Y., Tariq, A., Zeng, F., Sardans, J., Peñuelas, J., Zhang, Z. and Xu, M. (2022). “Fertile islands” beneath three desert vegetation on soil phosphorus fractions, enzymatic activities, and microbial biomass in the desert-oasis transition zone. Catena, 212, 106090. https://doi.org/10.1016/j.catena.2022.106090
Hang, S. B., Negro, G. J., Becerra, M. A., and Rampoldi, E. A. (2015). Suelos de Córdoba. Variabilidad de las propiedades del horizonte superficial. FCA, Universidad Nacional de Córdoba.
Karlin, M. S. (2012). Cambios temporales del clima en la subregión del Chaco Árido. Multequina, 21, 3-16. https://www.redalyc.org/pdf/428/42825278001.pdf
Karlin, M. S. (2013). Relaciones suelo-planta en el ecosistema Salinas Grandes, Provincia de Catamarca (Argentina). Tesis Doctoral. Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba. https://rdu.unc.edu.ar/handle/11086/1564
Karlin, M. S., Bernasconi Salazar, J., Cora, A., Sánchez, S. Arnulphi, S. and Accietto, R. (2019). Cambios en el uso del suelo: capacidad de infiltración en el centro de Córdoba (Argentina). Ciencia del Suelo, 37(2), 196-208. http://www.ojs.suelos.org.ar/index.php/cds/ article/view/435/245
Karlin, M. S., Coirini, R., Ringuelet, A., Salazar, J. B., Cora, A., Contreras, A., Bravo, M. B. and Bufia, E. (2021). Evaluación biofísica de islas de fertilidad en el Chaco Árido (Argentina). AgriScientia, 38(1), 1-13. https://doi. org/10.31047/1668.298x.v38.n1.30529
Karlin, M., Galán, R., Contreras, A., Zapata, R., Coirini, R. and Ruiz Posse, E. (2013). Exergetic model of secondary successions for plant communities in the Arid Chaco (Argentina). International Scholarly Research Notices, 2013, 945190. http://doi. org/10.1155/2013/945190
Karlin, M. S., Karlin, U. O, Coirini, R. O., Reati, G. J. and Zapata, R. M. (2013). El Chaco Árido. Editorial Encuentro.
Karlin, M. S., Zapata, R. M. and Coirini, R. O. (2021). Soil organic carbon and dead biomass pools in woodlands from Monte region (Argentina). Bosque, 42(1), 67-79. https://www.revistabosque.org/index.php/bosque/article/view/236
Kremers, J. and Boosten, M. (2018). Soil compaction and deformation in forest exploitation. American Journal for Alternative Agriculture, 7(1-2), 25-31. https://www.starobv.nl/wp-content/uploads/2020/02/Rap2018 soil compaction and deformation in forest exploitation.pdf
Lei, S. A. (2010). Benefits and costs of vegetative and sexual reproduction in perennial plants: a review of literature. Journal of the Arizona-Nevada Academy of Science, 42(1), 9-14. https://doi.org/10.2181/036.042.0103
Mahall, B. E. and Callaway, R. M. (1991). Root communication among desert shrubs. Proceedings of the National Academy of Sciences, 88(3), 874-876. https://doi.org/10.1073/pnas.88.3.874
Martino, R. D., Guereschi, A. B., Carignano, C. A., Sfragulla, J. A. and Bonalumi, A. A. (2020). Mapa geológico de la Provincia de Córdoba. Servicio Geológico Minero Argentino, Instituto de Geología y Recursos Minerales. https://repositorio.segemar.gov. ar/handle/308849217/4117
Mustapha, A. A., Abdulrahman, B. L., Dawaki, M. U. and Usman, A. (2022). Comparative determination of available phosphorus at different pH levels in Nigerian Savannah soils using Mehlich, Olsen and Bray Methods. Nigerian Journal of Soil Environmental Research, 21, 69-77.
Naldini, M. B., Harguindeguy, N. P. and Kowaljow, E. (2021). Soil carbon release enhanced by increased litter input in a degraded semi-arid forest soil. Journal of Arid Environments, 186, 104400. https://doi.org/10.1016/¡.¡aridenv. 2020.104400
Nelson, D. and Sommers, L. (1996). Total carbon, organic carbon and organic matter. In: Sparks, D. L. (Ed.). Methods of Soil Analysis Part 3. Chemical Methods (pp. 961-1010). ASA SSSA CSSA.
Ochoa-Hueso, R, Hernandez, R., Pueyo, J., Manrique, E. (2011). Spatial distribution and physiology of biological soil crusts from semi-arid central Spain are related to soil chemistry and shrub cover. Soil Biology and Biochemistry, 43(9), 1894-1901. https://doi.org/10.1016/¡.soilbio.2011.05.010
Passera, C. B., and Borsetto, O. (1986). Determinación “Índice de Calidad Específico”. In: Subcomité Asesor del Árido Subtropical Argentino (Ed.). Taller de arbustos forrajeros para zonas áridas y semiáridas (pp. 80-88). Orientación Gráfica.
Passera, C. B., Dalmasso, A. D. and Borsetto, O. (1986). Método de Point Quadrat Modificado. In: Subcomité Asesor del Árido Subtropical Argentino (Ed.j. Taller de arbustos forrajeros para zonas áridas y semiáridas (pp. 71-79). Orientación gráfica.
Potsch, S., and Arens, K. (1949). Sobre a ecologia da Selaginella sellowii Hieron. Lilloa, 20, 89-104. https:// www.lillo.org.ar/journals/index.php/lilloa/article/view/1426
Qu, L., Wang, Z., Huang, Y., Zhang, Y., Song, C. and Ma, K. (2018). Effects of plant coverage on shrub fertility islands in the Upper Minjiang River Valley. Science China Life Sciences, 61, 340-347. https://doi. org/10.1007/s11427-017-9144-9 Ridolfi, L., Laio, F. and D'Odorico, P. (2008). Fertility island formation and evolution in dryland ecosystems. Ecology and Society, 13(1), 5. https://www.istor.org/ stable/26267910
Riginos, C., Grace, J. B., Augustine, D. J. and Young, T. P. (2009). Local versus landscape-scale effects of savanna trees on grasses. Journal of Ecology, 97(6), 1337-1345. https://doi.org/10.1111/¡.1365-2745.2009.01563.x
Roos, P. C. and Allsopp, N. (1997). Soil nutrient ecology associated with Acacia sieberiana at different tree densities in a South African savanna. African Journal of Range & Forage Science, 14(2), 39-44. https://doi. org/10.1080/10220119.1997.9647918 Rossi, B. E. and Villagra, P. E. (2003). Effects of Prosopis flexuosa on soil properties and the spatial pattern of understorey species in arid Argentina. Journal of Vegetation Science, 14(4), 543-550. https://doi. org/10.1111/j.1654-1103.2003.tb02181.x Schafer, J. L., Mudrak, E. L., Haines, C. E., Parag, H. A., Moloney, K. A. and Holzapfel, C. (2012). The association of native and non-native annual plants with Larrea tridentata (creosote bush) in the Mojave and Sonoran Deserts. Journal of Arid Environments, 87, 129-135. https://doi.org/10.1016/Uaridenv. 2012.07.013 Sims, J. T (2000). Soil test phosphorus: Bray and Kurtz P-1. In: Pierzynski, G. M. Methods of phosphorus analysis for soils, sediments, residuals, and waters. Southern Cooperative Series Bulletin No. 396 (pp. 1314). North Carolina State University.
Teich, I., Cingolani, A. M., Renison, D., Hensen, I. and Giorgis, M. A. (2005). Do domestic herbivores retard Polylepis australis Bitt. woodland recovery in the mountains of Córdoba, Argentina? Forest Ecology and Management, 219(2-3), 229-241. https://doi. org/10.1016/¡.foreco.2005.08.048 Thompson, D. B., Walker, L. R., Landau, F H. and Stark,
L. R. (2005). The ¡nfluence of elevation, shrub species, and biological soil crust on fertility islands in the Mojave Desert, USA. Journal of Arid Environments, 61(4), 609629. https://doi.org/10.1016/Uaridenv.2004.09.013
Tongway, D. J. and Ludwig, J. A. (2005). Heterogeneity in arid and semiarid lands. In: Lovett, G. M., Jones, C., Turner, M. G., and Weathers, K. C. (Eds.) Ecosystem function in heterogeneous landscapes (pp. 189-205). Springer. https://doi.org/10.1007/0-387-24091-8 10
United Nations Convention to Combat Desertificaron (UNCCD). (2015). Integration of the sustainable development goals and targets into the implementation of the United Nations Convention to Combat Desertificaron and the Intergovernmental Working Group report on land degradation neutrality. Decision 3/COP.12. Report of the Conference of the Parties on Its Twelfth Session, Held in Ankara from 12 to 23 October 2015. https://www.unccd.int/official-documentscop-12-ankara-2015/3cop12
Varela, O., Varas, M., Rattalino, D., Crabbe, F. and Ordano, M. (2017). Ameliorative effects of nurse shrubs on soil chemical characteristics are driven by plant size in the Monte Desert. Arid Land Research and Management, 31(4), 418-430. https://doi.org/10.1080/15324982.201 7.1340359
Villagra, P. E., Giordano, C., Alvarez, J. A., Bruno Cavagnaro, J., Guevara, A., Sartor, C., Passera, C. B. and Greco, S. (2011). Ser planta en el desierto: estrategias de uso de agua y resistencia al estrés hídrico en el Monte Central de Argentina. Ecología Austral, 21(1), 29-42. https://ojs.ecologiaaustral.com. ar/index.php/Ecologia Austral/article/view/1294
Ward, D., Trinogga, J., Wiegand, K., du Toit, J., Okubamichael, D., Reinsch, S. and Schleicher, J. (2018). Large shrubs increase soil nutrients in a semiarid savanna. Geoderma, 310, 153-162. https://doi. org/10.1016/¡.geoderma.2017.09.023
Wood, M. H., and Carvalho, P. C. de F (2000). Defoliation patterns and herbage intake on pastures. In: Lemaire, G., Hodgson, J., Moraes, A. D., Carvalho, P. D. F and Nabinger, C. Grassland ecophysiology and grazing ecology. CABI Publishing.
Zarekia, S., Jafari, M., Arzani, H., Javadi, S. A. and Jafari, A. A. (2012). Grazing effects on some of the physical and chemical properties of soil. World Applied Sciences Journal, 20(2), 205-212.