10.31047/1668.298x.v1.n40.38788
Articulos
Effect of cumin seed (Cuminum cyminum L.) essential oil from Catamarca, Argentina, on the stored maize pests Sitophilus zeamais and Fusarium verticillioides
V. del V Quiroga
R. P Pizzolitto
M. P Zunino
J. S. Dambolena
J. M. Herrera
J. A. Zygadlo
1 Quiroga, V. del V. (ORCID: 0000-0002-1541-1342), Universidad Nacional de Catamarca, Facultad de Ciencias Exactas y Naturales. San Fernando del Valle de Catamarca, Catamarca, Argentina. Pizzolitto, R. P. (ORCID: 0000-0001-8321-0756), Instituto Multidisciplinario de Biología Vegetal (IMBIV-CONICET). Córdoba, Argentina. Universidad Nacional de Córdoba, Facultad de Ciencias Agropecuarias, Departamento de Recursos Naturales, Cátedra de Microbiología Agrícola. Facultad de Ciencias Exactas, Físicas y Naturales, Departamento de Química, Cátedra de Química Orgánica. Córdoba, Argentina. Zunino, M. P. (ORCID: 0000-0002-5320-0036), Dambolena, J. S. (ORCID: 0000-0001-8690-6564), Herrera, J. M. (ORCID: 0000-0001-7435-1856) and Zygadlo J. A. (ORCID: 0000-0001-9339-4464), Instituto Multidisciplinario de Biología Vegetal (IMBIV-CONICET). Córdoba, Argentina. Universidad Nacional de Córdoba, Facultad de Ciencias Exactas, Físicas y Naturales, Departamento de Química, Cátedra de Química Orgánica. Córdoba, Argentina. Correspondence to: rpizzolitto@imbiv.unc.edu.ar
SUMMARY
The essential oil composition of Cuminum cyminum L. from Catamarca province, Argentina, and its insecticide and antifungal activities were studied, with the major constituents detected by gas chromatography (GC) and gas chromatography-mass spectrometry (GC/MS) being: cuminaldehyde (20.58 %), Y-terpinene (20.43 %), p-cymene (17.35 %) and p-pinene (13.75 %). Insecticidal activity was tested against Sitophilus zeamais Motschulsky. The results showed that cumin oil lethal concentrations (LC) LC50 and LC95 values were 66.39 pL/L air and 370.14 pL/L air, respectively. Moreover, the essential oil had a repellent effect at 4 pL/L and 0.4 pL/L and an inhibition of acetylcholinesterase activity of 88.39 % and 47.75 % at concentrations of 9.2 and 2.3 mg/L, respectively. Antifungal activity against Fusarium verticillioides was tested at 250,500 and 1000 pL/L. For the highest concentration of cumin essential oil, the mycelia growth was inhibited by around 80 %. Lag phase and growth rate of F verticillioides was affected considerably and showed concentration dependence. The results obtained in this study revealed the possible use of cumin oil as a natural alternative in controlling S. zeamais and F verticillioides, the main pests of stored maize.
Key words: cuminaldehyde, insecticidal effect, repellence-attraction, antifungal activity, acetylcholinesterase.
RESUMEN
Se estudió la composición del aceite esencial de Cuminum cyminum L. de la provincia de Catamarca, Argentina, y su actividad insecticida y antifúngica. Los principales constituyentes detectados por GC y GC/MS fueron: cuminaldehído (20,58 %), Y-terpineno (20,43 %), p-cimeno (17,35 %) y p-pineno (13,75 %). Se evaluó la actividad insecticida contra Sitophilus zeamais Motschulsky. Los resultados mostraron valores de concentración letal (CL) CL50 y CL95 del aceite de comino de 66,39 pL/L y 370,14 pL/L de aire, respectivamente. Además, el aceite esencial tuvo un efecto repelente a 4 pL/L y 0,4 pL/L y una inhibición de la actividad acetilcolinesterasa de 88,39 % y 47,75 % a concentraciones de 9,2 y 2,3 mg/L, respectivamente. La actividad antifúngica contra Fusarium verticillioides se evaluó a 250, 500 y 1000 pL/L. En el caso de la concentración más alta de aceite esencial de comino, se observó una inhibición del desarrollo fúngico de aproximadamente 80 %. La fase de latencia y la tasa de crecimiento de F verticillioides se vieron afectadas considerablemente y fueron dosis-dependiente. Los resultados obtenidos en este estudio revelaron el posible uso del aceite de comino como alternativa natural en el control de S. zeamais y F verticillioides, principales plagas del maíz almacenado.
Palabras clave: cuminaldehído, efecto insecticida, repelencia-atracción, actividad antifúngica, acetilcolinesterasa.
INTRODUCTION
Cuminum cyminum L. (Apiaceae) is a shrub growing up to 40 cm high and cultivated for its seeds and essential oils since ancient times. Nowadays, cumin is found among the spices with the largest global marketing and is used in gastronomy and traditional medicine in countries such as India, Turkey, Iran, Pakistan and China. As a flavoring additive it has now been integrated in the western menu as a result of increased consumption of exotic or ethnic foods. To cover this higher demand for cumin seed and its essential oil, many producing countries should increase their crop areas and non-producers need to incorporate growing areas for the first time.
Argentina is among the most important South American exporters of cumin seeds, with a growing area of 5.95 square kilometers and a production of 900 Tons (Cameroni, 2012; Sánchez et al., 2020). Cumin growing areas in Argentina, traditionally represented by the provinces of Catamarca and Salta, have recently expanded to the provinces of La Rioja and Tucumán (Cameroni, 2012; Sánchez, 2012). A variety known as INTA Sumalao N°1 (Murua Carrizo, 2013) is the preferred type cultivated in Argentina. However, information concerning the composition of the essential oil of cumin cultivated in Argentina is scarce (Bandoni et al., 1991). From previous studies, the analysis of essential oils of cumin has always shown p-pinene; Y-terpinene; p-cymene, p-mentha-1,4-dien-7-al and cuminaldehyde to be the main compounds, regardless of their geographical origins (Bandoni et al., 1991; Beis et al., 2000; Jalali-Heravi et al., 2007; Li et al., 2009; Derakhshan et al., 2010; Oroojalian et al., 2010; Bettaieb et al., 2011; Gohari and Saeidnia, 2011). On the other hand, p-mentha-1,3-dien-7-al, phellandral, acoradiene and 2-caren-10-al are constituents that are not always present in some essential cumin oils (El-Sawi and Mohamed, 2002; Gachkar et al., 2007; Allahghadri et al., 2010; Bettaieb et al., 2011; Yeom et al., 2012; Kim et al., 2013; Kedia et al., 2014). The international standard ISO 9301 for cumin seed oil (C. cyminum L.) shows that the major constituents of the chromatographic profile are: p-mentha-1,3-dien-7-al; p-mentha-1,4-dien-7-al; and p-mentha-3-en-7-al, with cuminic aldehyde, p-pinene and Y-terpinene being the main components (ISO 9301).
Cumin olí, In addition to its flavouring properties, has also shown antifungal activity (Hashem et al., 2010; Gohari and Saeidnia, 2011; Kedia et al., 2014) and antimicrobial activity. Thus, Gachkar et al. (2007) have reported bioactivity against diverse microorganisms: they have observed an inhibitory effect on Escherichia coli, Staphylococcus aureus and Listeria monocytogenes growth. Sharifzadeh et al. (2016) found minimal inhibitory concentration (MIC) values ranged from 1000 to 2000 mg/mL for the Aspergillus and Penicillium species tested. In addition, repellent and insecticidal activities of cumin oils have been reported against the rice weevil Sitophilus oryzae (Chaubey, 2011; Kim et al., 2013). Thus, this pest control capacity of cumin oil could be used to avoid the application of synthetic pesticides to control an infestation of insects or to prevent the growth of fungi on the stored food. In corn kernel silos, two pests of great significance are S. zeamais (Motschulsky) (Coleoptera: Curculionidae) and F. verticillioides (Zunino et al., 2015; Peschiutta et al., 2022; Brito et al., 2022). The former is a primary pest of stored maize that feeds on healthy grains, with the damage generated by its presence favouring fungal infections and the occurrence of secondary metabolites. The latter pest is the main fungal pathogen of maize plant and major fumonisin B1 (FB1) producer (Chulze, 2010). In this investigation, we studied the C. cyminum essential oil composition from Catamarca (Argentina) and its fumigant insecticidal and antifungal activities against the maize weevil and F. verticillioides.
MATERIAL AND METHODS
Plant material and essential oil obtainment
C. cyminum L. seeds were collected within the territory of the province of Catamarca, Argentina, during 2008-2010. The study area was between longitude -27.39° and -28.46° and latitude -65.73° and -67.25°. After drying the seeds at room temperature (30 °C), the plant material was hydrodistilled for five hours in a full glass Clevenger type apparatus. The oil was then separated, dried using anhydrous sodium sulphate, and stored in dark glass bottles in a freezer at -4 °C until being used. Four samples of cumin oil were analysed in this study.
Essential oil analysis
The gas chromatography (GC) study was performed using a Perkin-Elmer 500 gas chromatograph equipped with a fíame ionization detector. A DB-5 fused silica capillary column (30 m x 0.25 mm, film thickness 0.25 |um, J&W Scientific Inc., Rancho Cordova, California, United States of America) was utilized with the working conditions being as follow: injector and detector temperature 250 °C and 265 °C, respectively; carrier gas, nitrogen; the oven temperature program 40 °C-250 °C, at a rate of 4 °C/min. The GC/MS equipment used was a Perkin Elmen Clarus 600 gas chromatograph coupled to a quadruple EI mass analyzer, with an apolar DB-5 capillary column (30 m x 0.25 mm, 0.25 |um film thicknesses). Helium was used as the carrier gas at a 0.8 ml/min flow rate. The mass spectrometer had an ionization potential of 70 eV, and the injector and the GC/MS interface temperatures were maintained at 290 °C and 300 °C, respectively. The mass spectra were recorded at 70 eV, and the mass range was from m/z 50 to 300. The ion source and the detector temperatures were maintained at 250 and 150 °C, respectively, and under a split condition (8:1), a 1 |ul sample was injected.
Identification of compounds
The compounds of essential oil were identified by their retention index (RI) relative to the C5-C24 n-alkanes (Sigma Aldrich, Buenos Aires, Argentina), by comparison of the mass spectra with those available in the NIST and Willey libraries and those of commercial standards by co-injection (Sigma Aldrich Co. Buenos Aires, Argentina). The peak area percentages were calculated from the capillary column without the use of FID response factors.
Insects
The insects were unsexed adults of S. zeamais Motschulky (Coleoptera: Curculionidae) whichwere kept under controlled conditions (26 °C, 60-70 % relative humidity and a 12 h: 12 h lighting regime (D:L)), and reared on whole maize grains (Food and Agriculture Organization, 1974). The adult weevils used in all the experiments were approximately two weeks old, and the bioassays were carried out in darkness and under temperature (28 °C) and relative humidity (70 %) control.
Fungal strain and inoculum preparation
The fungal strain employed in the antifungal tests was F verticillioides (Sacc) Niremberg (= F. moniliforme Sheldon teleomorph G. fujikuroi Sawada Ito ¡n Ito & Kimura Leslie and Summerel, 2006) strain M3125, which was provided by Dr. Robert Proctor from the United States Department of Agriculture (Agricultural Research Service, National Centre for Agricultural Utilization Research, Peoria, Illinois). Fungal conidia were harvested from 7-day old culture on Czapek-Dox agar medium employing aqueous solution. The conidial concentration (106 conidia/mL) was adjusted using a Neubauer chamber.
Insecticida! activity
The insecticidal activity against S. zeamais was evaluated by fumigant toxicity analysis as described by Huang et al. (2000) with some modifications. Concentrations corresponding to 18.75 to 300 pL/L air of essential oil doses were applied to Whatman filter paper disks (2 cm diameter), which were placed on the underside of the screw cap of the fumigation chambers (30 ml-glass vials). Then, ten weevils were placed in each chamber. After 24 h, ¡nsect mortality was verified, and the LC50 and LC95 values were calculated according to Finney (1971).
Five replicates were carried out per essential oil dose and the control treatment was performed under the same conditions without the addition of cumin oil.
Repellent/Attraction Bioactivity
A two-choice olfactometer was employed to evaluate the behavioral response of S. zeamais adults to cumin oil (Herrera et al., 2015). Two flasks (250 mL) were connected with a glass tube (30 cm x 1 cm of diameter) and in the middle (15 cm from the two flasks) a 1 cm x 1 cm small hole was made. The connections between the two flasks and the tube were sealed with rubber plugs, which were covered with parafilm to prevent gas leakage. The cumin oil (dissolved in n-hexane) was added to a filter paper of 2 cm diameter, which was placed ¡nside the treatment flask. The solvent (n-hexane) was placed ¡n a filter paper ¡n the control flask. Twenty insects, deprived of food for at least 24 h, were placed in the hole of the glass tube. After 2 h, we recorded the location of the insects in the flasks. Repellent/Attraction bioactivity assays were replicated five times.
A response index (RI) was calculated by the following equation: RI = (T - C)/Tot x 100. Where T is the number of insects responding to the treatment; C is the number of insects responding to the control; and Tot is the total number of insects released (Phillips et al., 1993). Positive (+) values of RI ¡ndicate attraction to the treatment. Negative (-) values of RI indicate repellence to the treatment.
In vitro AChE inhibition tests
The acetylcholinesterase (AChE) activity was evaluated by employing a 96-well microplate method proposed by Brühlmann et al. (2004), with modifications.The effect of different concentrations of C. cyminum EO (9.2 and 2.3 mg/L) on AChE activity was evaluated using S. zeamais adults (0.5 g, approximately 100 adults) homogenized in 5 mL of 0.1 M ice-cold phosphate buffer (pH 7.4) using a Teflon glass tissue homogenizer. Then, the homogenates were centrifuged (5000 rpm for 20 min at 0 °C) and supernatants were used as the enzyme source for AChE activity determination. A colorimetric method described by Ellman et al. (1961) was used to determine the inhibition of AChE, as a percentage calculated as: AChE inhibition % = (ODC - ODT/ODC) x 100, whereas ODC is the optical density of control and ODT is the optical density of the treatment. All the treatments were repeated three times.
Effect of essential oil on fungal growth
. verticillioides inhibition was tested by the plate dilution method using Czapek-dox agar. The culture medium was autoclaved (120 °C for 15 minutes) and cumin EO was added before cooling at 45 °C, to obtain concentrations of 250, 500 and 1000 pL/L. Then 10 pL of the conidia suspension, prepared as described above, was inoculated centrally in the Czapek-dox agar plates, and was incubated at 28 °C until the fungal growth reached the edge of the plate. The control treatment was prepared using Czapek-dox agar plates without the addition of EO and five replicates were performed per essential oil dose. The periodical measurement of two right-angled diameters of the colonies was used to determine the radial mycelial growth, colony diameters versus time were plotted, and the radial growth rates (mm/day) were evaluated from the slope by linear regression, with lag phase being determined as the abscissa from the growth rate curves.
Statistical analysis
Data were analyzed using InfoStat Software (Di Rienzo et al., 2017). Behavioral responses and anti-AChE activities were evaluated by Duncan's multiple range test at p < 0.05. The LC50 and LC95 values were obtained using Probit analysis (Finney, 1971). In the antifungal bioassays, data were evaluated through two-way variance analysis (ANOVA). Data normality was tested using the Shapiro-Wilk test. Comparisons between treatments were performed by the DGC (Di Rienzo, Guzmán and Casanoves) test (Di Rienzo et al., 2002). Results giving p < 0.05 values were considered significantly different.
RESULTS AND DISCUSSION
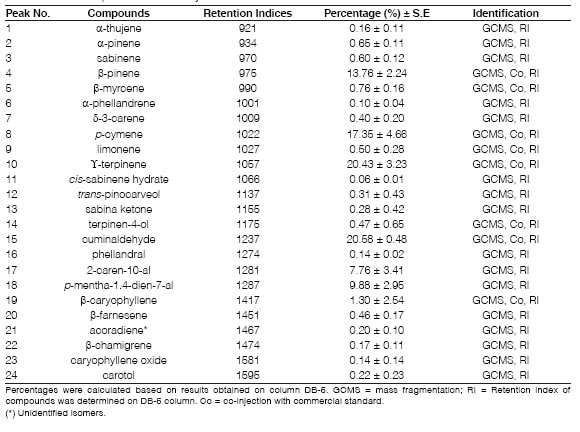
The essential oil composition obtained from cumin seeds is presented in Table 1. The cumin essential oil content, expressed on a dry basis, was 4.4 ± 0.4 ml per 100 g of ground dry cumin subjected to hydro distillation. The essential oil obtained from these cumin seeds were pale yellow and had a pungent odor.
As a result of our GC-MS studies, a total of 27 compounds were identified for the essential cumin oils, with p-pinene (13.75 %), p-cymene (17.35 %), Y-terpinene (20.43 %), cuminaldehyde (20.58 %), 2-caren-10-al and p-mentha-1,4-dien-7-al (9.88 %) that represents more than 89 % of the total compounds. The bibliography reports p-pinene, Y-terpinene, cuminal and p-mentha-1,4-dien-7-al being the major compounds of cumin oil using various extraction methods (Li et al., 2009; Allahghadri et al., 2010; Martinez-Velazquez et al., 2011; Abbdellaoui et al., 2019). Similar results were obtained in this investigation with cumin essential oil from Catamarca (Argentina). Moreover, our study also showed 20 compounds present at low amounts (>1.0 %). Several studies have reported the composition of essential oils is region dependent (Zygadlo, 2011; Lopez et al., 2012; Van Baren et al., 2015) and cumin oil is not an exception (El-Sawi and Mohamed, 2002; El-Kamali et al., 2009; Bettaieb et al., 2011). In this regard, when we compared the chemical composition of cumin essential oils from San Juan and La Rioja province (Argentina) reported by Bandoni et al. (1991) with that obtained in the present study from cumin seeds from Catamarca, some differences were observed. Bandoni et al. (1991) reported a high concentration of 1,4-p-menthadien-7-al (<19.4 %) and the presence of 1,3-p-menthadien-7-al, which is in agreement with ISO 9301, while our analysis showed a low concentration of p-mentha-1,4-dien-7-al (9.88 ± 2.95 %), with p-mentha-1,3-dien-7-al not being detected. However, some authors have suggested that p-mentha-1,3-dien-7-al is an artifact formed during the storage of ground seeds or in the distillation of oils (Bandoni et al., 1991; Sowbhagya et al., 2008).

Table 1: Chemical composition of Cuminum cyminum L. essential oil from Catamarca
The insecticidal, repellent and acetylcholinesterase inhibition activities of the cumin oil on adult maize weevil S. zeamais were tested, and the results of fumigant insecticide activity are shown in Table 2. The LC50 and LC95 were 66.39 pL/L air and 370.14 pL/L air, respectively, with the inhibition of AChE activity by cumin oil being 88.39 % and 47.75 % for concentrations of 9.2 and 2.3 mg/L. In addition, the behavioral response of S. zeamais to cumin essential oil revealed a repellent effect at 4 pL/L and 0.4 pL/L (Table 2). However, a minor repellency effect of cumin essential oil against S. zeamais was observed by Rosa et al. (2019). Earlier investigations have shown a significant insecticidal activity of cumin essential oil against S. oryzae and S. granarius (Abdelgaleil et al., 2009; Chaubey, 2011; Kim et al., 2013), but there is scarce bibliographic information on the insecticidal activity of cumin essential oil against S. zeamais (Rosa et al., 2019). The toxic effect of cumin oil on S. oryzae has been reported with the following LC50 values: 0.136 mL/mL air (Lashgari et al., 2014), 0.67 pL/cm3 (Chaubey, 2011) and 4.75 mg/L air (Kim et al., 2013). S. granarius reported 80 % mortality when exposed to 16 pL/L air of cumin essential oil (Ziaee et al., 2014); while the data of Rosa et al. (2019) revealed a low fumigant toxicity, 229.5 mg/L, against S. zeamais. Our results revealed an LC50 of 66.39 pL/L air for cumin oil from Argentina on S. zeamais. The differences observed in the lethal concentrations mentioned above could be due to a variation in the essential oil compositions and also to differences in responses among the Sitophilus species (Germinara et al., 2008; Niewiada et al., 2005).
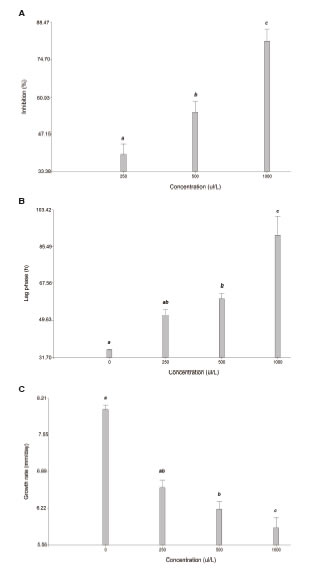
Figure 1: Cuminum cyminum L. antifungal effect on Fusarium verticillioides M3125 growth. A. Fungal growth inhibition (%). B. Lag phase (h). C. Growth rate (mm/day). Bars with different letters are statistically different (p < 0.05).
As cuminaldehyde, 2-caren-10-al, p-mentha-1,4-dien-7-al are the main carbonyl components of cumin oil (Jirovetz et al., 2005; Martinez-Velazquez et al., 2011; Ziaee et al., 2014) they may be responslble for the insecticidal activity. Related to this, the aldehyde compound concentration (cuminaldehyde, 2-caren-10-al and p-mentha-1,4-dien-7-al) of the cumin essential oil analyzed in the present study was 38.32 %, while the carbonyl content of the cumin oils studied by Kim et al. (2013) and Ziaee et al. (2014) was 17.67 % and 65.6 %, respectively. A previous report found that aldehydes had higher levels of insecticidal activity (Zunino et al., 2015), and the toxic effect of cuminal on S. oryzae has been reported with the following values of LC50: 1.12 mg/L air (Kim et al., 2013) and 71.4 mg/L air (Abdelgaleil et al., 2009). Although insecticidal activity may be more complex than just a simple inhibition of AChE activity, the terpenes ability to inhibit this enzyme is frequently a measure of these natural products insecticidal potency (Lopez and Pascual-Villalobos, 2010; 2015).
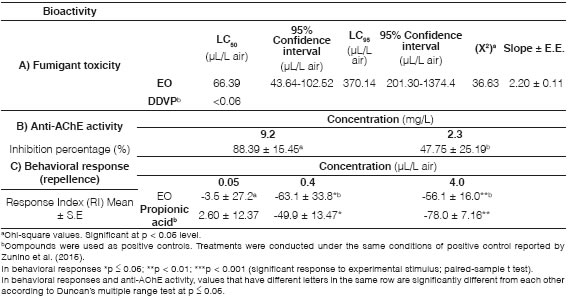
Table 2: Bioactivity of Cuminum cyminum L. essential oil from Catamarca on Sitophilus zeamais (A: Fumigant toxicity. B: Anti-AChE activity. C: Behavioral response)
In this study the effect of cumin oil on AChE activity in S. zeamais adults, was tested to identify this essential oil possible target site. C. cyminum oil from Catamarca, Argentina, showed a high inhibition rate (88.39 %) at a concentration of 9.3 mg/L, revealing a direct relationship between AChE activity and insecticidal ability. Although the inhibition of AChE activity by cumin oil may change with insect species (Abdelgaleil et al., 2009), the reported toxicity of cumin oils on AChE activity from S. oryzae was generally in agreement with the results of the present study (Abdelgaleil et al., 2009; Chaubey, 2011; Kim et al., 2013).
The antifungal activity of essential oil of cumin seeds from Catamarca (Argentina) against F. verticillioides M3125 are shown in Figure 1. The bioactivity was tested at 250,500 and 1000 pL/L and, at the highest concentration of cumin essential oil, the mycelia growth of F. verticillioides was inhibited by around 80%, while inhibitions of 55 % and 40 % were shown at 500 and 250 pL/L essential oil concentrations, respectively, thus revealing a dose dependent effect (Figure 1a). Moreover, our results showed that the F. verticillioides lag phase was critically affected by an increase of the cumin oil concentration (Figure 1b). The adaptation phase was slightly more than one day, which by increasing the concentration from 250 pL/L to 1000 pL/L extended this lag phase to three days with respect to the start of mold growth. It can be observed in Figure 1c that fungal growth was affected considerably with the greatest reduction observed at 1000 pL/L. Regarding antifungal activity, in the present study, cumin oil treatment resulted in the reduction of fungal growth, with the lag phase and growth rate showing significant differences with the control at concentrations of up to 500 ppm. These results are in agreement with the findings of Hashem et al. (2010) who reported C. cyminum essential oils having an antifungal effect against different species of Fusarium. Moreover, Mohammadpour et al. (2012) and Yooussef et al. (2016) demonstrated a biological activity against Aspergillus species.
CONCLUSION
In summary, the results obtained in this study indícate that the essential oil of C. cyminum seeds from Catamarca, rich in cuminaldehyde, Y-terpinene, p-cymene and p-pinene, has biological activity against S. zeamais and F verticillioides. These findings suggest that cumin oil can be used as a natural alternative to chemical pesticides to prevent S. zeamais infestation on stored corn, using concentrations of 4 |jl_ EO/L air to repel weevils. This study also suggests that employing cumin oil concentration superior to 1000 |jL EO/L induces significant inhibition of fungal and insecticidal activities.
ACKNOWLEDGEMENTS
Financial support for this work came from the following sources: Secretaría de Ciencia y Tecnología de la Provincia de Catamarca, SUCYTCA (PROCAICyT 2014-4), Universidad Nacional de Catamarca (SECyT-SPU), FONCyT (PICT 2019-2703; 2018-3697), Universidad
Nacional de Córdoba (SECyT) and CONICET (PIP 11220200102478). The authors also wish to express their gratitude to Dr. S. Fuentes, to Lic. L Favore, to Ing. G Alemmanno, to Prof. A Leiva, to Prof. L Montivero and to Tec. E Soriano. We also thank Dr. Marcela Palacio and Damian Barrionuevo (M.S.) for their technical assistance and Dr. Paul D. Hobson for revising the English language.
Fecha de recepción: 21/09/2022
fecha de aceptación: 07/07/2023
REFERENCES
Abbdellaoui, M., Bouhlali, E. D. T. and Rhaffari, L. E. (2019). Chemical composition and antioxidant activities of the essential oils of cumin (Cuminum cyminum) conducted under organic production conditions. Journal of Essential Oil Bearing Plants, 22(6), 1500-1508. https:// doi.org/10.1080/0972060X.2019.1699866
Abdelgaleil, S. A. M., Mohamed, M. I. E., Badawy, M. E. I. and El-Arami, S.A.A. (2009). Fumigant and contact toxicities of Monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. Journal of Chemical Ecology,35, 518-525. https://doi. org/10.1007/s10886-009-9635-3 Allahghadri, T., Rasooli, I., Owlia, P., Nadooshan, M. J., Ghazanfari, T., Taghizadeh, M. and Astaneh, S. D. A. (2010). Antimicrobial property, antioxidant capacity, and cytotoxicity of essential oil from cumin produced in Iran. Journal of Food Science,75, 54-61. https://doi. org/10.1111/j.1750-3841.2009.01467.x
Bandoni, A., Juárez, M. and Mizrahi, I. (1991). Contribución al estudio de las esencias de comino (Cuminum cyminum L.). Anuales de SAIPA, 32-49. Beis, S. H., Azcan, N., Ozek, T., Kara, M. and Baser, K. H. C. (2000). Production of essential oil from Cumin seeds. Chemistry of Natural Compounds,36, 265-268. https://doi.org/10.1007/BF02238331 Bettaieb, I., Bourgou, S., Sriti, J., Msaada, K., Limam, F. and Marzouk, B. (2011). Essential oils and fatty acids composition of Tunisian and Indian cumin (Cuminum cyminum L.) seeds: A comparative study. Journal of the Science of Food and Agriculture,91, 2100-2107. https://doi.org/10.1002/jsfa.4513
Brito, V. D., Achimón, F., Zunino, M. P., Zygadlo, J. A. and Pizzolitto, R. P. (2022). Fungal diversity and mycotoxins detected in maize stored in silo-bags: a review. Journal of the Science of Food and Agriculture, 102(7), 26402650. https://doi.org/10.1002/jsfa.11756
Brühlmann, C. Marston, A., Hostettmann, K., Carrupt, P. A. and Testa, B. (2004). Screening of non-alkaloidal natural compounds as acetylcholinesterase inhibitors. Chemistry & Biodiversity, 1(6), 819-29. https://doi. org/10.1002/cbdv. 200490064 Cameroni, M. G. (2012). Ficha del comino. Cuminum cyminum. L. Ministerio de Agricultura, Ganadería y Pesca. Subsecretaria de Valor Agregado y Nuevas Tecnologías. http://www.contenido/sectores/ aromáticas/productos/comino_2012_10Oct_11-pdf.
Chaubey, M. K. (2011). Fumigant toxicity of essential oils against rice weevil Sitophilus oryzae L. (Coleoptera: Curculionidae). Journal of Biological Sciences, 11(6), 411-416. https://doi.org/10.3923/jbs.2011.411.416 Chulze, S. N. (2010). Strategies to reduce mycotoxin levels in maize during storage: a review. Food Addit Contam Part A, 27(5), 651-657. https://doi. org/10.1080/19440040903573032 Derakhshan, S., Sattari, M. and Bigdeli, M. (2010). Effect of cumin (Cuminum cyminum) seed essential oil on biofilm formation and plasmid Integrity of Klebsiella pneumoniae. Pharmacognosy Magazine, 6(21), 5761. http://dx.doi.org/10.4103/0973-1296.59967 Di Rienzo, J. A., Casanoves, F., Balzarini, M. G., González, L., Tablada, M. and Robledo, C. W. InfoStat (versión 2017) Software. Córdoba, Argentina: Grupo InfoStat, FCA, Universidad Nacional de Córdoba. http://www. infostat.com.ar
Di Rienzo, J., Guzmán, A. and Casanoves, F. (2002). A multiple-comparisons method base on the distribution of the root node distance of a bionary tree. Journal of Agricultural Biological and Environmental Statistics,7, 1-14. https://doi.org/10.1198/10857110260141193 El-Kamali, H. H., Adam, S. I. Y., Adam, A. S., Abbakar, F. M. and Babikir, I. A. (2009). Aromatic plants from the Sudan: Part I. Chemical composition and antibacterial activity of Cuminum cyminum L. essential oil. Advances in Natural and Applied Sciences, 3(1), 1-4. https://www.researchgate.net/proflle/Shama-
Adam/publication/278963273_Aromatic_Plants_ from_the_Sudan_Part_I_Chemical_Composition_ and_Antibacterial_Activity_of_Cuminum_cyminum_L_ Essential_Oil/links/5588076f08ae65ae5a4d9582/ Aromatic-Plants-from-the-Sudan-Part-I-Chemical-Composition-and-Antibacterial-Activity-of-Cuminum-Cyminum-L-Essential-Oil.pdf
Ellman, G. L., Courtney, K. D., Andres, V. and Featherstone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, 7(2), 88-95. https://doi.org/10.1016/0006-2952(61)90145-9
El-Sawi, S. A. and Mohamed, M. A. (2002). Cumin herb as a new source of essential oils and its response to foliar spray with some micro-elements. Food Chemistry, 77(1), 75-80. https://doi.org/10.1016/S0308-8146(01)00326-0
Food and Agriculture Organization (FAO). (1974). Boletín Fitosanitario de la FAO. Método provisional para gorgojos adultos importantes en cereales almacenados con malatión o lindano. Método N°15 FAO, 22, 127-137
Finney, D. J. (1971). Probit analysis (3 ed.). Cambridge Unlverslty Press.
Gachkar, L., Yadegari, D., Rezaei, M. B., Taghizadeh, M., Astaneh, S. A. and Rasooli, I. (2007). Chemical and biological characteristics of Cuminum cyminum and Rosmarlnus offlclnallsessentlal olls. Food Chemistry, 102(3), 898-904. https://doi.org/10.1016/j. foodchem.2006.06.035
Germinara, G. S., De Cristofaro, A. and Rotundo, G. (2008). Behavioral responses of adult Sitophilus granariusto individual cereal volatiles. Journal of Chemical Ecology 34, 523-529. https://doi.org/10.1007/s10886-008-9454-y
Gohari, A. R. and Saeidnia, S. (2011). A review on phytochemistry of Cuminum cyminum seeds and its Standards from Field to Market. Pharmacognosy Journal, 3(25), 1-5. https://doi.org/10.5530/pj.2011.25.1
Hashem, M., Moharam, A. M., Zaied, A. A. and Saleh, F E. M. (2010). Efflcacy of essential olls In the control of cumin root rot disease caused by Fusarium spp. Crop Protection, 29(10), 1111-1117. https ://doi. org/10.1016/j.cropro.2010.04.020
Herrera, J. M., Pizzolitto, R. P, Zunino, M. P, Dambolena, J. S. and Zygadlo, J. A. (2015). Effect of fungal volatile organic compounds on a fungus and an insect that damage stored maize. Journal of Stored Product Research, 62, 74-80. https://doi.org/10.1016/j.jspr.2015.04.006
Huang, Y., Lam, S. L. and Ho, S. H. (2000). Bioactivities of essential oil from Elletaria cardamomum(L.) Maton. to Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst). Journal of Stored Product Research, 36(2), 107-117. https://doi.org/10.1016/ S0022-474X(99)00040-5
ISO 9301, a.n.d. Oil of cumin seeds (Cuminum cyminum). https://www.iso.org/standard/34045.html
Jalall-Heravl, M., Zekavat, B. and Sereshtl, H. (2007). Use of gas chromatography-mass spectrometry combined with resolution methods to characterize the essential oil components of Iranian cumin and caraway. Journal of Chromatography A,1143(1-2), 215-226. https://doi. org/10.1016/j.chroma.2007.01.042 Jirovetz, L., Buchbauer, G., Stoyanova, A. S., Georgiev, E. V. and Damianova, S. T. (2005). Composition, quality control and antimicrobial activity of the essential oil of cumin (Cuminum cyminumL.) seeds from Bulgaria that had been stored for up to 36 years. International Journal of Food Science and Technology, 40(3), 305-310. https://doi.org/10.1111/j.1365-2621.2004.00915.x Kedia, A., Prakash, B., Mishra, P K., Chanotiya, C. S. and Dubey, N. K. (2014). Antlfungal, antlaflatoxlgenlc, and lnsectlcldal efflcacy of spearmlnt (Mentha splcata L.) essential oil. International Biodeterioration & Biodegradation, 89, 29-36. https://doi.org/10.1016/j. ibiod.2013.10.027
Klm, S.W., Kang, J. and Park, I. K. (2013). Fumlgant toxicity of Apiaceae essential oils and their constituents against Sitophilus oryzae and their acetylcholinesterase inhibitory activity. Journal of Asia-Pacific Entomology16(4), 443-448. https://doi. org/10.1016/j.aspen.2013.07.002 Lashgari, A., Mashayekhi, S., Javadzadeh, M. and Marzban, R. (2014). Effect of Mentha piperita and Cuminum cyminum essential oil on Tribolium castaneum and Sitophilus oryzae. Archives of Phytopathology and Plant Protection, 47(3), 324-329. https://doi.org/10.1080/03235408.2013.809230 Leslie, J. and Summerel, B. A. (2006). Species concepts in Fusarium. In: Leslie, J and Summerell, B. A. (Eds.) The Fusarium Laboratory Manual (88-95). Hoboken. https://doi.org/10.1002/9780470278376
Li, X. M., Tian, S. L., Pang, Z. C., Shi, J. Y., Feng, Z. S. and Zhang, Y. M. (2009). Extraction of Cuminum cyminum essential oil by combination technology of organic solvent with low boiling point and steam distillation. Food Chemistry, 115(3), 1114-1119. https://doi.org/10.1016/j.foodchem.2008.12.091 Lopez, M. D. and Pascual-Villalobos, M. J. (2010). Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Industrial Crops and Products, 31(2), 284-288. https://doi.org/10.1016/j. indcrop.2009.11.005
Lopez, M. D. and Pascual-Villalobos, M. J. (2015). Are monoterpenoids and phenylpropanoids efficient inhibitors of acetylcholinesterase from stored product insect strains? Flavour and Fragrance Journal, 30, 108-112. https://doi.org/10.1002/ffj.3220
Lopez, S., Lima, B., Aragón, L., Ariza Espinar, L., Tapia, A., Zacchino, S., Zygadlo, J., Feresin Egly, G. and López, M. L. (2012). Essential Oil of Azorella cryptantha Collected in Two Different Locations from San Juan Province, Argentina: Chemical Variability and Anti-Insect and Antimicrobial Activities. Chemistry and Biodiversity, 9(8), 1452-1464. https://doi.org/10.1002/ cbdv.201100319
Martinez-Velazquez, M., Castillo-Herrera, G. A., Rosario-Cruz, R., Flores-Fernandez, J. M., Lopez-Ramirez, J., Hernandez-Gutierrez, R. and Del Carmen Lugo-Cervantes, E. (2011). Acaricidal effect and chemical composition of essential oils extracted from Cuminum cyminum, Pimenta dioica and Ocimum basilicum against the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Parasltology Research,108, 481-487. https://doi.org/10.1007/s00436-010-2069-6
Mohammadpour, H., Moghimipour, E., Rasooli, I., Hadi, M., Astaneh, S. A., Moosaie, S. S. and Jalili, Z. (2012). Chemical composition and antifungal activity of Cuminum cyminum L. essential oil from Alborz Mountain against Asperillus species. Jundishapur Journal of Natural Pharmaceutical Products, 7(2), 50-55. https://www.ncbi.nlm.nih.gov/pmc/articles/ PMC3941858/
Murua Carrizo, F. (2013). Efecto de la época y densidad de siembra de comino (Cuminum cyminum L.) sobre el rendimiento, sus componentes y la calidad de semilla. Horticultura Argentina, 30, 9-17. http://agrarias. unca.edu.ar/wp-content/uploads/2018/Revista%20 de%20Divulgaci%C3%B3n%20T%C3%A9cnica%20 Agr%C3%ADcola%20y%20Agroindustrial/Revista-54-El-comino-en-Catamarca-Argentina.pdf
Niewiada, A., Nawrot, J., Szafranek, J., Szafranek, B., Synak, E., Jele, H. and Wa sowicz, E. (2005). Some factors affecting egg-laying of the granary weevil (Sitophilus granarius L.). Journal of Stored Product Research,41(5), 544-555. https://doi.org/10.1016/j. jspr.2004.11.001
Oroojalian, F., Kasra-Kermanshahi, R., Azizi, M. and Bassami, M. R. (2010). Phytochemical composition of the essential oils from three Apiaceae species and their antibacterial effects on food-borne pathogens. Food Chemistry, 120(3), 765-770. https://doi.org/10.1016/j. foodchem.2009.11.008
Peschiutta, M. L., Achimón, F., Brito, V. D., Pizzolitto, R. P., Zygadlo, J. A. and Zunino, M.P. (2022). Fumigant toxicity of essential oils against Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae): a systematic review and meta-analysis. Journal of Pest Science, 95, 1037-1056. https://doi.org/10.1007/ s10340-021-01457-1
Phillips, T W., Jiang, X. L., Burkholder, W. E., Phillips, J. K. and Tran, H. Q. (1993). Behavioral responses to food volatiles by two species of stored-product Coleoptera, Sitophilus oryzae (Curculionidae) and Tribolium castaneum (Tenebrionidae). Journal of Chemical Ecology, 19, 723-734. https://doi.org/10.1007/BF00985004
Rosa, J. S., Oliveira, L., Sousa, R. M. O. F, Escobar, C. B. and Fernandes-Ferreira, M. (2019). Bioactivity of some Apiaceae essential oils and their constituents against Sitophilus zeamais (Coleoptera: Curculionidae).
Bulletin of Entomological Research, 110(3), 406-416. https://doi.org/10.1017/S0007485319000774
Sánchez, H. H., 2012.El cultivo de comino: aspectos a considerar para la producción de comino. INTA. https://inta.gob.ar/sites/default/files/script-tmp-inta-_ sanchez_comino.pdf
Sánchez, H. H., Sabadzija, G. N. and Zamboni, M. (2020). Análisis técnico económico de modelos productivos de Comino y Anís. Contexto, potencialidades y limitantes para su desarrollo en Argentina. (Estudio Socioeconómico de la sustentabilidad de los sistemas de producción y recursos naturales N° 17). Ministerio de Agricultura Ganadería y Pesca. Argentina. INTA. https://repositorio.inta.gob.ar/ xmlui/bitstream/handle/20.500.12123/6876/INTA_ CRCatamarca_LaRioja_EEACatamarca_Sanchez_H_ Analisis_tecnico_economico_modelos_productivos. pdf?sequence=1&isAllowed=y
Sharifzadeh, A., Javan, A. J., Shokri, H., Abbaszadeh, S. and Keykhosravy, K. (2016). Evaluation of antioxidant and antifungal properties of the traditional plants against foodborne fungal pathogens. Journal de Mycologie Médicale, 26, e11-e17. https://doi.org/10.1016/j.mycmed.2015.11.002
Sowbhagya, H. B., Sathyendra Rao, B. V. and Krishnamurthy, N. (2008). Evaluation of size reduction and expansion on yield and quality of cumin (Cuminum cyminum) seed oil. Journal of Food Engineering, 84(4), 595-600. https://doi.org/10.1016/j. jfoodeng.2007.07.001
Van Baren, C. M., Elechosa, M. A., Di Leo Lira, P., Retta, D. S., Juaréz, M. A., Martínez, A. J., Molina, A. M. and Bandoni, A. L. (2015). Acantholippia seriphioides: Chemical biodiversity of wild populations from the Cuyo region in Argentina. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromaticas,14, 3341. https://www.redalyc.org/pdf/856/85632845004.pdf
Yeom, H. J., Jung, C. S., Kang, J., Kim, J., Lee, J. H., Kim, D. S., Kim, H. S., Park, P. S., Kang, K. S. and Park, I. K. (2012). Insecticidal and acetylcholine esterase inhibition activity of asteraceae plant essential oils and their constituents against adults of the German cockroach (Blattella germanica). Journal of Agricultural and Food Chemistry,63(8), 2241-2248. https://doi.org/10.1021/jf505927n
Yooussef, M. M., Pham, Q., Achar, P. N. and Sreenivasa, M. Y. (2016). Antifungal activity of essential oils on Aspergillus parasiticus isolated from peanuts. Journal of Plant Protection Research, 56(2), 139-142. https:// doi.org/10.1515/jppr-2016-0021
Ziaee, M., Moharramipour, S. and Mohsenifar, A. (2014). MA-chitosan nanogel loaded with Cuminum cyminum essential olí for efflclent management of two stored product beetle pests. Journal of Pest Science, 87, 691-699. https://doi.org/10.1007/s10340-014-0590-6
Zunino, M. P., Herrera, J. M., Pizzolitto, R. P, Rubinstein, H. R., Zygadlo, J. A. and Dambolena, J. S. (2015). Effect of Selected Volatiles on Two Stored Pests: The Fungus Fusarlum verticillioides and the Maize Weevll Sithophiluszeamais. Journal of Agricultural and Food Chemistry,63(35), 7743-7749. https://doi.org/10.1021/ acs.jafc.5b02315
Zygadlo, J. (2011). Los aceites esenciales. Aspectos básicos de su química y biosíntesis. Composición de los aceites esenciales de plantas aromáticas argentinas. In Zygadlo, J. (Ed.), Aceites esenciales. Química, ecología, comercio, producción y salud (1153). Universitas.